Analgesia and Anesthesia for Labor and Delivery
Authors
INTRODUCTION
Anesthesiologists and anesthesia play a critical role in the modern labor and delivery unit. Cesarean deliveries require the participation of an anesthesia provider in the care of the parturient (and by extension, the fetus). In addition, the anesthesiologist plays a critical role in the care of many high-risk parturients. As part of the perinatal team, anesthesiologists contribute knowledge in the areas of invasive monitoring and the diagnosis and treatment of the extremes of hemodynamic and respiratory instability. Finally, the number of parturients choosing neuraxial (epidural and spinal) labor analgesia for uncomplicated labor and vaginal delivery has increased from 21% and 55% in small and large hospitals, respectively, in 1992 to 57% and 77% in 2001.1 At the author's institution, the rate of neuraxial labor analgesia in 2009 was 90%.
PHYSIOLOGIC AND ANATOMIC CHANGES DURING PREGNANCY AND LABOR: ANESTHETIC IMPLICATIONS
Many physiologic and anatomic changes occur during pregnancy, labor and delivery, and the postpartum period that directly impact the administration of analgesia and anesthesia (Table 1).
Table 1. Physiologic and anatomic changes during pregnancy: anesthetic implications
Change | Anesthetic Implications |
| Circulation | |
Hyperdynamic: increased reliance on sympathetic nervous system | Increase in incidence and severity of hypotension after neuraxial analgesia/anesthesia |
Capillary engorgement, increase in airway edema | Increased incidence of difficult airway |
Aortocaval compression | More profound hypotension with parturient in the supine position after induction of neuraxial analgesia/anesthesia |
Metabolism and respiration | |
Increase in O2 requirement and CO2 production | Greater risk of desaturation after induction of general anesthesia |
Decrease in FRC | Greater risk of desaturation after induction of general anesthesia |
Gastrointestinal system | |
| Increased progestrone levels | Decreased tone of lower esophageal sphincter, increased risk of aspiration pneumonitis |
| Displacement of the intraabdominal esophagus into thorax | Decreased tone of the lower esophageal sphincter, increased risk of aspiration pneumonitis |
Increased intraabdominal (gastric) pressure | Increased risk of aspiration pneumonitis |
Endocrine | |
Increased progesterone levels | Increased pain threshold |
Nervous system | |
Increase in ß-endorphin concentration | Increased tolerance to pain |
Increased susceptibility to local anesthetics | Decrease in local anesthetic dose requirements |
Anatomic changes in the spinal column | Decrease in local anesthetic dose requirements |
Increased susceptibility to CNS depressants | Decrease in dose requirements for general anesthetics and adjuvants |
Pharmacokinetics | |
Altered volume of distribution | Change in drug pharmacokinetics |
Altered protein binding of drugs | Change in drug pharmacokinetics |
Increased renal blood flow | Change in drug pharmacokinetics |
Altered hepatic microsomal enzyme activity | Change in drug pharmacokinetics |
FRC, functional residual capacity; CNS, central nervous system.
Circulation
In general, the cardiovascular system is hyperdynamic during pregnancy. However, arterial responsiveness to vasopressors is reduced in pregnancy in both in vivo and in vitro animal models.2, 3 In contrast, the venous pressor response increases in pregnancy.4 Indeed, hemodynamic stability becomes more dependent on sympathetic nervous system activity as pregnancy progresses,5 probably as a result of dependence on venous return. Neuraxial analgesia/anesthesia-induced sympathetic blockade in the term parturient results in a more marked decrease in blood pressure compared with that in nonpregnant control subjects.5
Capillary engorgement of the oral, nasal, pharyngeal, and tracheal mucosa may contribute to a markedly increased incidence of difficult endotracheal intubation in obstetric patients.6 Nasotracheal intubation should be avoided because of the risk of epistaxis. An overall increase in vascularity, particularly in the pelvis, may contribute to the altered rate of absorption of local anesthetics injected for nerve blocks, and increase the risk of inadvertent intravascular injection.
AORTOCAVAL COMPRESSION
Term parturients in the supine position experience a 10–20% decrease in cardiac output secondary to aortocaval compression.7 Compression of the vena cava begins as early as 13–16 weeks' gestation8 and is nearly complete in the majority of parturients at term. In the lateral decubitus position, there is partial caval compression,9 but collateral circulation through the azygous system maintains venous return.10 There is partial compression of the aorta in the supine position that is completely relieved by the lateral position.11
Objective parameters of fetal well-being, including fetal scalp capillary blood pH, transcutaneous pO2, and fetal heart rate pattern, deteriorate when parturients labor in the supine position.12, 13 This is true whether or not neuraxial analgesia/anesthesia-induced sympathetic blockade is present. However, the adverse effects may be more profound in the parturient with sympathetic blockade. In addition, aortocaval compression may occur in the lateral decubitus position when maximum lumbar flexion is assumed during positioning for neuraxial procedures. Cardiac output is more likely to decrease in this position compared to the sitting position.14
Metabolism and respiration
Oxygen requirements and carbon dioxide production increase 60% during pregnancy.15 Minute ventilation increases by 45% primarily as a result of an increase in tidal volume.16 In addition to the change in minute ventilation, the most important lung volume/capacity change is a 20% decrease in functional residual capacity (FRC) (i.e., volume of the lung at the end of normal expiration).16
During labor and delivery, minute ventilation may increase up to 300%, oxygen consumption increases by 75%, and the PaCO2 decreases to as low as 10–15 mmHg.17 Maternal aerobic oxygen requirements exceed oxygen consumption during labor and delivery, resulting in a progressive rise in maternal lactate levels.18
The increased maternal oxygen requirement and decreased functional residual capacity (FRC) (decreased oxygen reserve) have profound implications for the induction of general anesthesia in the parturient. Because of concerns of pulmonary aspiration (see later), parturients are almost never ventilated by mask during the induction of anesthesia. On induction, an intravenous sedative hypnotic (e.g., thiopental) is administered, immediately followed by a neuromuscular blocking agent. Patients remain apneic until the endotracheal tube is placed. Provided there is no difficulty with intubation, the apneic period is approximately 1 minute. During this period, the pO2 in the parturient falls at more than twice the rate of nonpregnant women.19 If oxygen is administered before the induction of anesthesia, resulting in a pO2 close to 500 mmHg, the parturient becomes hypoxemic after 3 minutes of apnea compared to 7 minutes in nonpregnant women.20 Increased minute ventilation and decreased FRC also increase the rate of uptake of inhaled anesthetic agents.
Finally, high spinal or epidural anesthesia is associated with a further decrease in FRC;21 therefore, the parturient is at increased risk, compared to the nonpregnant patient, for having hypoxemia develop during neuraxial anesthesia.
Gastrointestinal system
Several changes in the gastrointestinal system result in the admonition that a “parturient is always a full stomach”. The gravid uterus displaces the stomach, often resulting in the displacement of the stomach into the thorax. There is a reduction in lower esophageal sphincter tone secondary to anatomic displacement22 and relaxation of the sphincter by progesterone.23 In addition, intragastric pressure rises. Together, these changes result in a reduced gastroesophageal barrier pressure and the well-known clinical complaint of heartburn.
Gastric emptying, as measured by several techniques, including acetaminophen absorption,24, 25, 26, 27 epigastric impedence,28 applied potential tomography,29 and ultrasonography,26, 27 is unchanged in pregnancy. However, labor significantly slows gastric emptying.28, 30 Furthermore, opioids administered by any route, including systemic,28 epidural,31, 32 or intrathecal,33 slow gastric emptying even more. In the immediate postpartum period (2–48 hours), gastric emptying does not appear to be delayed unless the parturient has received opioid analgesia.24, 25, 28, 29, 34
ANESTHETIC IMPLICATIONS
Pulmonary aspiration and failed intubation were responsible for 48% of anesthesia-related maternal mortality in the United States between 1979 and 1990.35 Indeed, Mendelson's syndrome, or pulmonary aspiration, was first reported in parturients receiving general anesthesia.36 Ollson37 reported that the incidence of pulmonary aspiration is higher in patients undergoing cesarean delivery (1 in 661) than in the general population undergoing surgery (1 in 2131). In addition, the incidence of aspiration is more than fourfold higher for emergency surgery compared with that of elective surgery.38 Aspiration often is associated with difficult intubation.39 These data support the premise that neuraxial anesthesia is safer for the parturient.
If general anesthesia is necessary, appropriate precautions are taken. The patient is most at risk for aspiration during induction and emergence from general anesthesia. Aspiration of acidic, rather than neutral, liquid results in more severe pathology.36 Histamine 2 (H2)-receptor antagonists reliably increase gastric pH by decreasing gastric acid production.37 H2-receptor antagonists, however, do not neutralize acid already present in the stomach. Therefore, a nonparticulate antacid (e.g., sodium citrate) is indicated before the induction of general anesthesia. A nonparticulate antacid is used because aspiration of particulate antacid may cause symptoms of aspiration pneumonitis.40
Many anesthesiologists also add metoclopramide to the aspiration prophylaxis cocktail. Metoclopramide increases lower esophageal sphincter tone41 and reduces gastric volume by increasing gastric motility.42
General anesthesia in the parturient is almost always induced by rapid sequence (see later). Mask ventilation is avoided, as air inadvertently introduced into the stomach increases the risk of aspiration. Similarly, deep “conscious” sedation is avoided in the parturient, as this may be associated with obtundation of protective airway reflexes. Before elective cesarean delivery, the American Society of Anesthesiologists' (ASA) Guidelines for Obstetrical Anesthesia recommend that patients undergo a fasting period of 2 h for clear liquids and 6–8 hours for solids (depending on fat content of the food).43
A related controversial issue is maternal oral intake during labor. Although laboring parturients who eat have larger gastric volumes than do parturients who fast,44 an association between eating during labor and an increased risk of aspiration has not been shown. Proponents of eating argue that oral intake improves patient comfort and satisfaction45 and prevents maternal ketoacidosis.44 Pulmonary aspiration is a rare event. Opponents of feeding argue that one cannot predict which parturient will require emergency surgery. Although the risk of aspiration is low, the morbidity and mortality are high. Mild ketosis has not been shown to adversely affect outcome, and a minority of patients find fasting stressful.45 The ASA Guidelines recommend that oral intake of modest amounts of clear liquid be allowed for uncomplicated laboring patients, although patients with additional risk factors for aspiration (e.g., morbid obesity, diabetes, difficult airway), or at increased risk for operative delivery may have further restriction of oral intake determined on a case-by-case basis.43 Solid foods should be avoided in laboring patients. These recommendations have been endorsed by the American College of Obstetricians and Gynecologists (ACOG).46
Endocrine
Progesterone concentration rises throughout pregnancy and declines abruptly after delivery of the placenta.47 The increase in plasma progesterone may influence a number of physiologic processes, including response to pain (see later).
Central and peripheral nervous system
Anatomic and physiologic changes in the nervous system alter responses to pain and susceptibility to both general and neuraxial anesthesia. Pregnancy-induced neurohumoral changes may alter responses to pain. In a rat model, the pregnancy-induced increased concentration of plasma β-endorphin was associated with an increased tolerance to visceral stimulation, and this effect was reversed by naloxone.48 Pregnancy is associated with lower plasma substance P concentrations49 and higher cerebrospinal fluid (CSF) progesterone levels.50
Nerves from pregnant animals (including humans) appear more susceptible to local anesthetic blockade. In intact rats51, 52 and humans,53, 54 pregnancy enhanced the effect of central and peripheral local anesthetics. Proposed mechanisms of enhanced neural blockade during pregnancy include hormone-related changes in the actions of spinal cord neurotransmitters, potentiation of the analgesic effect of endogenous analgesic systems, increased permeability of the neural sheath, and other pharmacodynamic or pharmacokinetic differences between pregnant and nonpregnant women.55 The extent of epidural local anesthetic blockade is increased during early pregnancy,53 a phenomenon explained by altered sensitivity to local anesthetics, as opposed to gross changes in spinal column anatomy.
As pregnancy progresses, anatomic changes in the mother may affect neuraxial anesthesic techniques. The epidural and vertebral foraminal veins are enlarged, resulting in an increased risk of intravascular placement of an epidural catheter and a decreased egress of epidural anesthetic agents from the epidural space. Engorged epidural veins are associated with a decrease cerebral lumbosacral CSF volume.56 An increase in intra-abdominal pressure is also associated with decreased CSF volume.57 In volunteers, decreased lumbosacral CSF volume, as measured by magnetic resonance imaging, was associated with increased sensory blockade after spinal anesthesia.58 The specific gravity of CSF is lower in pregnancy and this alters the distribution of hyperbaric and hypobaric anesthetic solutions.59
Together, these changes result in a 25% reduction in the segmental dose requirement for spinal anesthesia50, 60 and a similar segmental dose reduction for epidural anesthesia.53
The CNS and cardiac toxicity of lidocaine61 and ropivacaine62 are not altered by pregnancy. Although an earlier study concluded that the plasma bupivacaine concentration necessary for cardiac toxicity was markedly decreased in the pregnant sheep,63 a more recent study does not support this conclusion.64
Just as peripheral nerves are more susceptible to local anesthetic during pregnancy, the parturient also is more susceptible to CNS depressants. The minimum alveolar concentration of volatile halogenated anesthetic agents (the median effective dose) is decreased 30% during pregnancy.65 Similarly, there is increased sensitivity to thiopental.66
Pharmacokinetics
Pregnancy alters disposition of drugs by several mechanisms. Volume of distribution may be altered. For example, the elimination half-life of thiopental is markedly prolonged secondary to a large increase in the volume of distribution.67 Plasma protein concentration decreases, leading to altered drug binding.68 For example, lidocaine is less protein bound during pregnancy, resulting in a higher free fraction in the blood.69 Increased renal blood flow and glomerular filtration and altered hepatic microsomal activity change renal and hepatic drug clearance.70
Uteroplacental unit
UTEROPLACENTAL BLOOD FLOW
Uterine blood flow (UBF) increases markedly during pregnancy, from 50 to 100 mL/min before pregnancy to 700–900 mL/min at term. There is a generalized decrease in vascular sensitivity to endogenous and exogenous vasopressors; however, the diminution in response may vary between uterine and systemic vessels. The altered vascular reactivity in pregnancy may be secondary to (1) changes in receptor number or function, (2) changes in metabolism or clearance of drugs, (3) changes in release of endogenous vasoactive substances, or (4) changes in sensitivity to vasoactive substances.71 UBF is related to uterine perfusion pressure and vascular resistance:
![]()
UBF is not autoregulated. Any decrease in uterine arterial pressure (systemic hypotension), increase in venous pressure (caval compression, uterine contraction, Valsalva maneuver), or increase in uterine vascular resistance (increase in uterine vasoconstriction relative to systemic vasoconstriction) decreases UBF. The net effect on UBF of any therapeutic intervention depends on relative changes in systemic and uterine vessels as they affect the determinants of UBF.
EFFECT OF NEURAXIAL ANALGESIA/ANESTHESIA ON UTERINE BLOOD FLOW
Neuraxial analgesia/anesthesia may affect UBF, both positively and negatively, by a number of mechanisms.71 Pain causes activation of the sympathetic nervous system. In pregnant ewes, acute stress increases plasma norepinephrine levels by 25% and decreases UBF by 50%.72 Labor pain also is associated with a marked increase in epinephrine concentration,73, 74 which has been associated with an increased incidence of abnormal fetal heart rate patterns.75 Labor analgesia is associated with a marked reduction in circulating catecholamines.73, 74 Pain also causes maternal hyperventilation and hyperventilation may cause a decrease in UBF.76
Neuraxial anesthesia-induced sympathetic blockade does not appear to alter UBF in normal parturients in the absence of hypotension.77 In the setting of severe pre-eclampsia, epidural analgesia with local anesthetic actually may increase intervillous blood flow.78
Neuraxial analgesia/anesthesia may adversely affect UBF primarily as a result of sympathetic blockade-induced maternal hypotension. Maternal hypotension decreases uterine artery pressure and increases uterine vascular resistance secondary to reflex release of vasoconstrictors.71 Neuraxial analgesia has been associated with uterine tachysystole resulting in a decrease in UBF (see later).
Unintentional intravenous injection of local anesthetic may decrease UBF by several mechanisms. A test dose containing epinephrine often is administered through the epidural catheter to rule out inadvertent intravenous placement of the catheter. If the catheter is in a vein, the intravenous epinephrine may cause transient maternal tachycardia and hypertension. In gravid ewes, intravenous epinephrine also causes a transient decrease in UBF.79 The epidural administration of local anesthetic solutions containing epinephrine does not appear to affect UBF in the healthy parturient.80
The amount of local anesthetic contained in a normal test dose does not affect uterine perfusion if injected into a vein,81 nor does the amount of local anesthetic absorbed from the epidural space after epidural anesthesia. However, higher concentrations of local anesthetics, achieved after inadvertent intravenous injection or after a paracervical block (see later), may cause uterine tachysystole, a decrease in UBF, and subsequent fetal bradycardia.71
EFFECT OF GENERAL ANESTHESIA ON UTEROPLACENTAL BLOOD FLOW
Anesthetic agents and adjuvants, in general, have no direct effect on UBF. Hypotension associated with general anesthesia may adversely affect UBF. The typical rapid sequence induction of general anesthesia for cesarean delivery (induction agent, followed by neuromuscular blocking agent, followed by laryngoscopy and skin incision) often is accompanied by a catecholamine surge that may cause a decrease in UBF.82 Mechanical hyperventilation decreases UBF.83, 84 It is unclear whether this is a result of hypocarbia or a direct result of mechanical hyperventilation (resulting in decreased venous return and decreased cardiac output).83
The placenta and the fetus
The anatomy and physiology of the placenta are discussed in detail elsewhere in this text. Whether anesthetic agents cross the placenta depends on a number of factors, including drug concentration and electrochemical gradients across the placenta, molecular weight, lipid solubility, degree of ionization, membrane surface area and thickness, maternal and fetal blood flow, placental binding and metabolism, and degree of maternal and fetal protein binding.85 Most drugs that parturients receive during the administration of analgesia/anesthesia cross the placenta. This includes volatile anesthetic gases, nitrous oxide, sedative/hypnotics, opioids, local anesthetics, antihypertensive agents, and vasopressors.85 Neuromuscular blocking agents and most anticholinesterases are charged molecules and have limited placental transfer. The effects of individual anesthetic agents on the fetus/neonate are discussed below.
ANALGESIA FOR LABOR AND VAGINAL DELIVERY
Labor pain, a form of acute pain, is perceived by many women as very severe or intolerable.86 The optimal analgesic for labor provides pain relief for first- and second-stage labor but otherwise has minimal effect on the mother or baby. Neuraxial labor analgesia (epidural, spinal, or combined spinal-epidural [CSE] analgesia) currently is the most effective method of labor and delivery analgesia. The side effect profile of these techniques is acceptable to most women and obstetricians. Current research aims to decrease the incidence and severity of side effects while maintaining excellent analgesia.
The pain of parturition
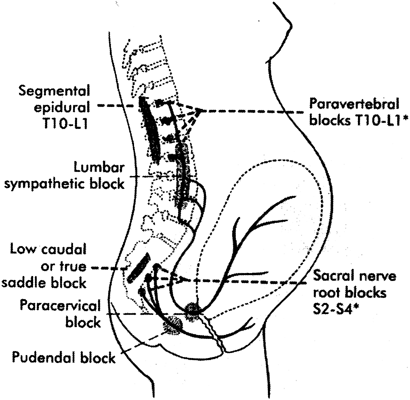
Parturition pain has both a visceral and somatic component. Visceral stimulation occurs as the cervix and lower uterine segment dilate. Early labor pain (latent phase and early active phase) is primarily visceral and occurs during uterine contractions. Afferent impulses are transmitted via sensory nerves (myelinated Aδ and unmyelinated C fibers) that pass through the paracervical nerve plexus (Fig. 1). The nerves accompany sympathetic nerves to the inferior, middle, and superior hypogastric and then celiac plexus. They enter the lumbar sympathetic chain and then pass with the T10, T11, T12, and L1 white rami communicates to the posterior spinal roots to synapse with neurons in the dorsal horn of the spinal cord. Pain often is referred to other areas innervated by these spinal segments, including skin, abdominal muscles, and the back.87
The somatic component of labor pain is caused by distension, inflammation, and tissue injury of the pelvic joints, vagina, pelvic floor, and perineum. This pain occurs as the fetus descends in the birth canal during the late first stage and second stage of labor. Afferent impulses are transmitted via the pudendal nerves through the sacral plexus to the spinal cord at S2, S3, and S4. The pudendal nerves also supply motor innervation to the pelvic floor and perineum.
The visceral and somatic components of labor pain can be blocked at various levels by one or more nerve blocks (see Fig. 1). For example, epidural analgesia can block the visceral, somatic, or both components of labor pain, depending on the spinal levels blocked. A lumbar sympathetic block alleviates visceral labor pain and a pudendal block alleviates somatic pain.
Analgesic requirements increase as labor progresses. The late active phase of labor (transitional stage) is the most painful period of labor. Minimum local analgesic concentration, or the median effective concentration, increases with advancing cervical dilation.88 A combined dose of intrathecal sufentanil and bupivacaine has a longer duration of action in early labor compared with late labor.89
Neuraxial labor analgesia
In addition to being the most effective form of labor analgesia,90 neuraxial analgesia has other salutary effects on maternal and fetal well-being. Effective analgesia prevents maternal hyperventilation during labor. Hyperventilation and hypocarbia may decrease UBF.76 In addition, hypocarbia causes a left shift in the maternal oxyhemoglobin dissociation curve, causing oxygen to be more tightly bound and decreasing the transfer of oxygen across the placenta.91 Finally, hyperventilation during contractions may be followed by periods of maternal hypoventilation between contractions, resulting in maternal and fetal hypoxemia.13, 92 Neuraxial analgesia results in a decrease in maternal oxygen consumption.93 Compared to systemic meperidine analgesia, epidural analgesia is associated with higher maternal oxygen saturation.94 Most parturients and fetuses are not harmed by this hyperventilation/hypoventilation cycle and increase in oxygen consumption. However, neuraxial analgesia may be advantageous for fetuses with marginal uteroplacental circulation and function.95
The increase in cardiac output associated with labor is attenuated by neuraxial analgesia.96 The neuraxial analgesia-associated decrease in systemic vascular resistance (SVR) may be advantageous to pre-eclamptic parturients and fetuses.78, 97
Neuraxial analgesia results in attenuation of the stress response during labor. The normal increase in maternal plasma beta-endorphin concentrations is attenuated by epidural analgesia.98 Circulating norepinephrine and epinephrine levels decrease after neuraxial analgesia,73, 74 and this may have a beneficial effect on fetal heart rate patterns.75 Fetal catecholamines probably facilitate adaptation to extrauterine life and are not affected by neuraxial analgesia.99, 100 Blockade of adrenergic-induced lipolysis results in lower free fatty acid levels in women who receive neuraxial analgesia.101 Finally, neuraxial analgesia is associated with lower maternal,102, 103 fetal, and neonatal lactic acid levels.18, 104
Neuraxial analgesia and the progress of labor
There has been much controversy as to whether neuraxial labor analgesia adversely affects the progress of labor and method of delivery. Early investigators noted that neuraxial analgesia appeared to be an effective treatment for dysfunctional labor.105, 106 However, more recently, there has been concern that neuraxial labor analgesia may prolong labor and increase the rate of operative delivery. Indeed, many observational studies show an association between neuraxial analgesia, prolonged labor, and operative delivery. However, association does not necessarily mean a cause-and-effect relationship. Painful labor may be a marker for longer, more difficult labors. Independent of neuraxial analgesia, women with early, and more severe, pain have a higher incidence of instrumental deliveries and abnormal fetal heart rate patterns.107
Observational studies have many limitations. Management of labor is not controlled. Many studies have included both nulliparous and parous parturients. Oxytocin administration may not be standardized. Cesarean delivery rates differ markedly among institutions, obstetricians,108 and insured versus uninsured patients.109
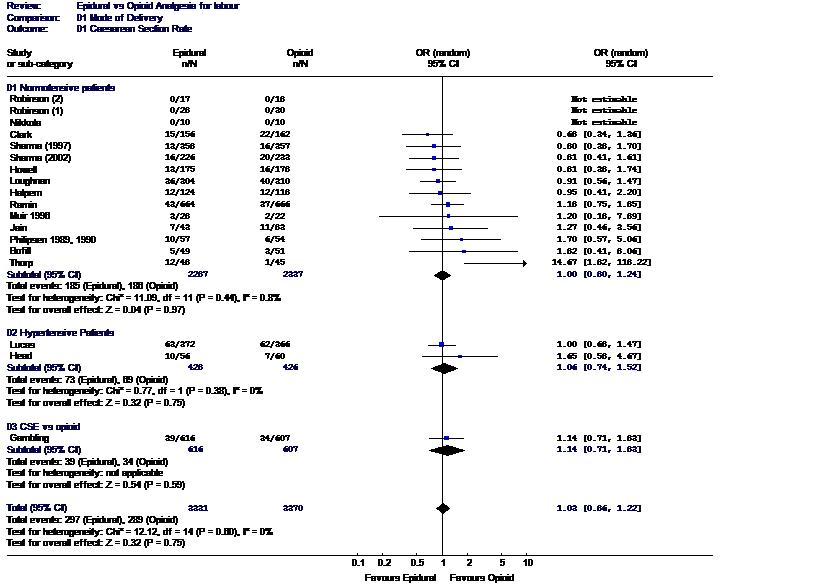
Randomized controlled trials addressing the issue of whether neuraxial analgesia increases the risk of cesarean delivery are difficult to perform. Blinding is impossible; therefore, standard labor management protocols must be rigidly followed or the study results may be biased. Neuraxial labor analgesia is superior to other forms of analgesia, and therefore, a significant number of patients may cross over study groups. Despite these limitations, a number of prospective, randomly assigned studies have been performed. A meta-analysis of these studies (over 6600 subjects) concluded that neuraxial analgesia does not increase the risk of cesarean delivery (Fig. 2).110 Retrospective studies comparing the rate of cesarean delivery immediately before and after the institution of on-demand neuraxial analgesia (impact studies) provide another method of studying this question. In all such studies, dramatic increases in the rate of neuraxial analgesia in a short period of time have had no impact on the rate of cesarean delivery for dystocia.111, 112
Another concern is whether early labor (latent phase) initiation of neuraxial analgesia adversely affects the mode of delivery and duration of labor. Several studies have addressed this issue by randomizing nulliparous who request analgesia in early labor (cervical dilation <4 cm) to neuraxial analgesia or systemic opioid analgesia.113, 114, 115, 116 Women randomized to the systemic opioid group received neuraxial analgesia when their cervix was dilated to ≥4 cm. These studies, and a meta-analysis,117 concluded that early labor neuraxial analgesia does not increase the rate of cesarean delivery compared to early systemic opioid analgesia. Additionally, the first stage of labor was shorter in women randomized to receive early neuraxial analgesia compared to systemic opioid.113, 114
Study results differ as to whether neuraxial analgesia increases the need for oxytocin augmentation.118, 119, 120, 121, 122 Variability in study results may result from differences in labor management protocols (e.g., use of active management of labor).119 Fluid management before initiation of epidural analgesia may influence uterine activity.123, 124 In addition, most studies compare epidural analgesia to systemic opioids (control group). The effect of systemic opioids and other forms of analgesia on the progress of labor are not known.
Studies also differ as to whether neuraxial analgesia affects the duration of the first stage of labor. Two meta-analyses came to different conclusions.110, 125 Sharma et al.125 concluded that the first stage of labor was significantly longer in women randomized to neuraxail compared to systemic labor analgesia (8.1 ± 5 h vs. 7.5 ± 5 h, P = 0.011), whereas Halpern and Leighton110 concluded there was no signficant difference (weighted mean difference (WMD) 24 min [95% CI −4 to 54 min], P = NS). The absolute difference between groups in both analyses was approximately one half hour. There is no evidence that this modest prolongation of the first stage of labor adversely affects the mother or neonate.
A final question is the effect of neuraxial analgesia on the second stage of labor. Mode of vaginal delivery has not been studied as the primary outcome variable, as these studies are difficult to execute and analyze for several reasons. Indications for instrumental vaginal delivery vary and are difficult to control. All neuraxial analgesia is not the same. Epidural administration of less-concentrated local anesthetic solutions (often containing opioid) minimizes the density of maternal motor block and is associated with a lower rate of instrumental deliveries compared to more concentrated solutions.126, 127 Some parturients are instructed to begin pushing when the cervix is fully dilated; others are instructed to begin pushing when they feel the urge or when the fetal head is on the perineum. A meta-analysis of studies comparing delayed to immediate (upon full cervical dilation) pushing concluded that there was no difference in the incidence of spontaneous vaginal delivery (RR 0.92 [95% CI 0.84–1.10], P = 0.10) or second stage cesarean delivery;128 however, the rate of rotational or mid-pelvic instrumental deliveries was significantly less in women with delayed pushing.
Most studies support the conclusion that the second stage of labor is prolonged in the presence of neuraxial analgesia.110, 125 Indeed, the ACOG defines a prolonged second stage differently in parturients with labor analgesia (in nulliparas: 2 h without neuraxial analgesia and 3 h with neuraxial analgesia).129 Studies suggest that a longer second stage is, in itself, not harmful to mother or neonate as long as the fetal heart rate pattern is reassuring and there is ongoing descent of the fetal head.128
EPIDURAL ANALGESIA
Lumbar epidural analgesia has been the mainstay of neuraxial labor analgesia. Placement of an epidural catheter allows analgesia to be maintained until after delivery. Randomized controlled trials consistently show that pain scores are lower and patients are more satisfied with epidural analgesia compared to other forms of analgesia.90, 118, 120, 121, 130, 131 There are few absolute contraindications to neuraxial analgesia. They include patient refusal or inability to cooperate, frank coagulopathy, uncorrected maternal hypovolemia, soft tissue or skin infection at site of needle placement, frank sepsis without prior antibiotic administration, and increased intracranial pressure secondary to a mass lesion. The specific epidural analgesic technique should be tailored to individual patient needs. The risks and benefits of epidural analgesia should be discussed with each parturient, preferably early in the labor process.
Lumbar epidural analgesia is initiated in either the sitting or lateral position. The epidural space is located with a 17- or 18-gauge needle. A flexible catheter is passed through the needle approximately 4 cm into the epidural space. The needle is removed and the catheter is secured. A test dose may be administered to rule out intrathecal or intravascular catheter placement. Analgesia is initiated by bolus injection through the needle, catheter, or both. Analgesia can be maintained with intermittent bolus injections, continuous infusion, or patient controlled epidural analgesia (PCEA). The catheter is removed after delivery when there is no further need for analgesia/anesthesia.
The ideal neuraxial analgesic technique would provide rapid onset of analgesia, effective analgesia with minimal motor block, minimal risk of maternal toxicity, minimal placental transfer and effects on the fetus, and long duration of action. Local anesthetics have been the mainstay of epidural analgesia for many years. There are advantages and disadvantages to using different local anesthetic agents. Bupivacaine and ropivacaine are the most commonly used local anesthetics for labor analgesia. Low concentrations (0.125% or less) provide excellent analgesia with minimal motor block. Both drugs are highly protein bound with minimal placental transfer,132 and duration of analgesia is approximately 2 hours. However, it may take 20 minutes to reach peak effect. Lidocaine has a faster onset than bupivacaine, but the duration of analgesia is shorter. It is less protein bound and therefore has a higher umbilical vein/maternal vein ratio than bupivacaine.133 However, controlled studies have found no difference in neonatal neurobehavioral scores between epidural lidocaine, bupivacaine, and 2-chloroprocaine.134, 135 2-chloroprocaine, an ester local anesthetic, has a rapid onset, but duration of analgesia is less than 45 minutes, limiting its routine use for labor analgesia. The half-life in maternal and fetal blood is less than 1 minute.136 It is most useful for rapidly extending epidural anesthesia for urgent or emergency operative delivery.
Opioids often are added to local anesthetics because they interact synergistically to provide analgesia.137 The combination allows for a decreased concentration of local anesthetic, thus decreasing motor block. Opioids administered into the epidural or subarachnoid space block visceral pain signals by binding to presynaptic and postsynaptic opioid receptors in the dorsal horn of the spinal cord.138 Neuraxial opioids provide excellent analgesia for early labor, when pain is primarily visceral in nature, without sympathetic or motor block. The addition of local anesthetics is necessary to block somatic stimuli later in labor.
Epidural opioids alone can provide moderate analgesia for early labor, but the necessary dose is accompanied by bothersome side effects (e.g., pruritus, nausea, vomiting, neonatal respiratory depression). Combining local anesthetics with opioids allows for effective analgesia while minimizing the undesirable side effects.139 In addition, onset of analgesia is more rapid and duration of analgesia is longer with the addition of opioid to local anesthetics.140
The lipid soluble opioids, fentanyl and sufentanil, often are most common combined with bupivacaine or ropivacaine. They have a rapid onset (5–10 minutes).140, 141 Their short duration of action (60–90 minutes)140 is overcome by maintaining analgesia with a continuous epidural infusion or patient-controlled epidural anesthesia (PCEA). Doses commonly used for epidural analgesia initiation and maintenance have been shown to be safe for both the mother and neonate.142, 143 In contrast, morphine (a water-soluble opioid) has a much slower onset (30–60 minutes) and a longer duration of action (12–24 hours).144 It is generally not used for continuous neuraxial analgesia because of its long latency.
Epidural analgesia may be maintained with intermittent bolus injection or continuous epidural infusion. Patient-controlled epidural analgesia is a modification of both techniques. Continuous epidural infusions result in reduced need for bolus injections145, 146, 147, 148, 149 and increased patient satisfaction.149, 150 Continuous infusion usually is accompanied by the gradual onset of perineal analgesia, obviating the need for a bolus dose at delivery. In studies comparing bolus injection to continuous infusion of bupivacaine, continuous infusion results in a higher total bupivacaine dose.145, 149, 150, 151 However, the infusion of lower-concentration bupivacaine at a higher rate may result in similar analgesia with less motor block and no increase in total dose.147, 150
PCEA allows for both a continuous epidural infusion and patient-titrated bolus injections. PCEA resulted in greater patient satisfaction152, 153 and a lower average hourly dose of bupivacaine.153, 154, 155
COMBINED SPINAL-EPIDURAL ANALGESIA
Combined spinal-epidural analgesia (CSE) has become increasingly popular in the past decade. CSE is a method of initiating neuraxial labor analgesia. After identifying the epidural space with an epidural needle, the anesthesiologist passes a small gauge spinal needle through the epidural needle and punctures the dura. A low dose of opioid, or opioid combined with local anesthetic, is injected into the subarachnoid space (spinal dose). The spinal needle is withdrawn and an epidural catheter is threaded through the epidural needle. Analgesia is maintained via the epidural catheter, as with traditional epidural analgesia.
There are advantages and disadvantages to CSE compared with traditional epidural analgesia (Table 2). Initiation of analgesia with an intrathecal opioid provides excellent analgesia for early labor without motor block or a sympathectomy. This is ideal for patients who wish to ambulate or for those who might poorly tolerate an acute sympathectomy. The effective opioid dose is significantly less than for systemic or epidural administration. Therefore, systemic absorption is minimal as are direct fetal effects. Another advantage is the rapid onset of analgesia. Complete analgesia occurs within 3–5 minutes.156 Finally, the addition of bupivacaine to a lipid-soluble opioid results in sacral analgesia within several minutes. Sacral analgesia is difficult to accomplish after a single lumbar epidural dose of local anesthetic. Therefore, CSE analgesia provides more complete analgesia for women in advanced stages of labor.
Table 2. Epidural versus combined spinal-epidural analgesia for labor and delivery
Epidural | Combined Spinal-Epidural | |
Onset | 10–15 min | 2–5 min |
Motor block | Mild to none | None with opioid-only dose |
Sacral block | Delayed 1–2 h | Immediate |
Sympathectomy | Present | None with opioid-only dose |
Epidural catheter | Able to determine that catheter is functional within 15–30 min | May not know whether catheter is functonal for 1–2 h |
Pruritus | Lower incidence, less severe | High incidence |
Fetal bradycardia | Comparable risk | Possibly higher risk |
PDPH | Comparable risk | Comparable risk |
PDPH, postdural puncture headache
There are several undesirable side effects of CSE analgesia. The incidence of pruritus is higher with intrathecal versus epidural opioids.127, 157, 158 Gastric emptying may be delayed more in patients who receive intrathecal versus epidural or systemic opioids.33
Another potential drawback of CSE analgesia is that it will be unclear for 1–2 hours after initiation of analgesia as to whether the epidural catheter is properly located in the epidural space (spinal analgesia must regress before functional epidural analgesia can be ascertained). Therefore, if it is important to have a functioning epidural catheter in place (e.g., in the presence of a worrisome fetal heart rate pattern or an anticipated difficult airway), then CSE analgesia may not be the technique of choice.
Several potential side effects of CSE analgesia are of concern. Fetal bradycardia not associated with maternal hypotension occurs after initiation of both epidural and CSE analgesia and the risk may be increased with CSE compared with epidural analgesia (see later). Respiratory depression or arrest has been reported after the intrathecal injection of sufentanil in laboring women.159 The risk appears to be increased when intrathecal opioids are administered before or after systemic opioids, and the risk is dose dependent.160
SPINAL ANALGESIA
In general, single-shot spinal analgesia is not useful for laboring patients because of its limited duration of action. It may be used in parturients who require analgesia/anesthesia shortly before anticipated delivery and in institutions in which resources are limited and continous epidural analgesia is not possible. Continuous spinal analgesia currently is not practical for most parturients. The available catheters (essentially epidural catheters) require a large-gauge introducer needle to place, and are therefore associated with an unacceptably high incidence of postdural puncture headache. However, the placement of a continuous spinal catheter is a management option in patients with unintentional dural puncture with an epidural needle.
CAUDAL ANALGESIA
Continuous caudal epidural analgesia is used infrequently in modern obstetric anesthesia practice. It is technically more difficult to place a caudal than lumbar epidural catheter. Large volumes of local anesthetic are required for first-stage analgesia and result in higher maternal plasma concentrations of drug. There is a risk of needle/catheter misplacement and direct injection into the fetus.
OTHER DRUGS FOR NEURAXIAL ANALGESIA
Other neuraxial drugs have been investigated in the search for the perfect analgesic technique. Epinephrine potentiates analgesia when added to epidural bupivacaine161, 162 or spinal bupivacaine/sufentanil.163 Epinephrine enhances local anesthetic-induced motor blockade. Stimulation of spinal cord postsynaptic α2 receptors produces analgesia by a different (cholinergic) mechanism than local anesthetics or opioids,164 and this may be one mechanism by which epinephrine enhances analgesia. Recent studies have investigated the role of epidural or spinal clonidine (α2 agonist) and neostigmine (anticholinesterase that increases spinal acetylcholine levels) in labor analgesia.165
OTHER NERVE BLOCKS
Neuraxial analgesia is the most effective and flexible analgesic technique for labor and delivery. However, some parturients may not be candidates for neuraxial analgesia or may not want it. Other nerve blocks may provide acceptable, albeit less-flexible, analgesia (Table 3).
Table 3. Other nerve blocks for labor and delivery analgesia
Block | Clinical Use | Advantage | Disadvantages/Side Effects/Complications |
Spinal (saddle block) | Instrumental vaginal delivery anesthesia | Rapid onset analgesia with perineal motor block | Single shot, not continuous |
Caudal block | Labor and delivery analgesia | Another access to epidural space | Technically more difficult than lumbar epidural |
Useful for patients with lumbar spine fusion | Requires large volume of local anesthetic to provide labor analgesia to T10 level | ||
Paracervical block | Early–mid 1st stage labor analgesia | No motor block | Not continuous |
Risk of fetal bradycardia | |||
Lumbar sympathetic block | Early–mid 1st stage labor analgesia | No motor block, speeds labor | Not continuous. Requires bilateral injections |
Useful for patients with lumbar spine fusion | Technically more difficult to learn | ||
Pudendal block | 2nd stage analgesia; instrumental vaginal delivery anesthesia | Performed by obstetrician before delivery | Not continuous |
Complications rare | |||
Perineal infiltration | Episiotomy or repair anesthesia | Technically simple | No motor relaxation |
Performed by the obstetrician as needed | Complications rare |
PARACERVICAL BLOCK
A paracervical block stops transmission of visceral afferent nerve impulses from the uterus and cervix through the paracervical, or Frankenhäuser's, ganglia. Advantages include excellent analgesia for the first stage of labor, before fetal descent, without somatic sensory or motor block. However, the block is not continuous and it does not relieve somatic pain caused by distension of the pelvic floor, vagina, or perineum.
The block is performed with the patient in lithotomy position with left uterine displacement. The obstetrician injects 5–10 mL of dilute local anesthetic solution by introducing the needle through the vagina into the left and right lateral vaginal fornix to a depth of 2–3 mm. Aspiration and incremental injection are recommended.
The choice of local anesthetic is controversial. In the United States, the manufacturers of bupivacaine state that it is contraindicated for paracervical block because it may be associated with a high rate of adverse neonatal outcome (see later).166 2-chloroprocaine has been advocated for paracervical block because its short intravascular half-life may limit adverse maternal and neonatal effects. Studies addressing this issue have too few patients to show a statistically significant improvement in outcome with 2-chloroprocaine.167, 168 Lidocaine 1% is the most commonly used local anesthetic for paracervical blocks.
Serious maternal complications are uncommon. They include systemic local anesthetic toxicity from inadvertent intravascular injection or rapid systemic absorption, postpartum neuropathy secondary to direct sacral plexus trauma or from hematoma formation,169 or retropsoal or subgluteal abscesses.170
Serious perinatal complications, including death, may result from paracervical block. Inadvertent direct fetal scalp injection can result in fetal systemic local anesthetic toxicity.171 This seems more likely to occur with advanced cervical dilation (greater than 8 cm).172
Fetal bradycardia is the most common fetal complication. It usually develops within 2–10 minutes after injection and resolves within 5–10 minutes. The reported incidence ranges from 0% to 70%.172 The etiology of fetal bradycardia is unclear. Most likely, fetal bradycardia results from a decrease in uteroplacental or fetoplacental perfusion secondary to local anesthetic-induced uterine tachysystole173 or uterine/umbilical artery vasoconstriction.174
Recommendations to reduce complications include the following:
- Perform the block only in parturients with no evidence of uteroplacental insufficiency.
- Perform the block when the cervix is dilated less than 8 cm.
- Monitor uterine activity and fetal heart rate continuously.
- After injecting local anesthetic on one side, wait 5–10 minutes before injecting anesthetic on the second side.
- Consider using 2-chloroprocaine.172
LUMBAR SYMPATHETIC BLOCK
A paravertebral lumbar sympathetic block interrupts transmission of visceral afferent nerve impulses from the uterus and cervix at the level of the L-2 to L-3 sympathetic chain. Similar to a paracervical block, it provides analgesia for the first stage but not the second stage of labor. Disadvantages include the following:
- The technique is not continuous.
- It is technically more difficult to learn and perform and requires bilateral injections.
Advantages include the following:
- It is associated with less fetal bradycardia than a paracervical block.
- It provides first-stage analgesia without any motor block.
- It is useful for patients with previous back surgery.175
- It does not adversely affect the progress of labor.176, 177 In fact, the course of labor appears accelerated compared to epidural analgesia.177
PUNDENDAL BLOCK
The pudendal nerve, which includes somatic fibers from S-2, S-3, and S-4, is the primary source of sensory innervation of the lower vagina, vulva, and perineum. It also provides motor innervation to the perineal muscles and the external anal sphincter. Thus, bilateral pudendal block alleviates pain during the second stage of labor resulting from vaginal, vulvar, and perineal distention. It provides satisfactory analgesia for spontaneous vaginal and low- or outlet-forceps delivery, but not mid-forceps delivery or exploration of the upper vagina, cervix, or uterine cavities. Bilateral success rate may be as low as 50%.178
The pudendal nerve can be blocked via the transperineal or transvaginal route. Most obstetricians in the United States use the transvaginal route immediately before delivery. However, earlier pudendal nerve blocks (just before or after complete cervical dilation) provide better analgesia and do not increase the incidence of instrumental delivery.179 This also allows for a repeat block, should an initial block fail.
The transvaginal pudendal block is performed through a needle guide. The needle is introduced through the vaginal mucosa and sacrospinous ligament, just medial and posterior to the ischial spine. Usually, 7–10 mL of local anesthetic solution is injected on each side. The pudendal artery lies close to the nerve; therefore, aspiration before and during injection is imperative. Rapid maternal absorption of local anesthetic occurs, resulting in peak levels in maternal venous and fetal scalp capillary blood between 10 and 20 minutes after injection.179 Lidocaine and 2-chloroprocaine often are used, with the same advantages and disadvantages as for paracervical block. Dilute concentrations (lidocaine 1% or 2-chloroprocaine 2%) are preferable, thus minimizing the risk of toxic maternal concentrations of local anesthetic.
Maternal and fetal complications of pudendal nerve block are rare. Maternal complications include (1) local anesthetic toxicity from direct intravascular injection or absorption of an excessive dose of local anesthetic; (2) vaginal, ischiorectal, or retroperitoneal hematomas;180 or (3) subgluteal or retropsoal abscesses.170 Fetal complications include fetal trauma, direct fetal injection, of local anesthetic, or both.181
PERINEAL INFILTRATION
Perineal infiltration often is used immediately before delivery to provide anesthesia for an episiotomy or repair. It provides no motor relaxation. Several milliliters of local anesthetic are injected into the posterior fourchette. In one study, after a mean dose of 79 ± 3 mg lidocaine, rapid maternal absorption and fetal transfer resulted in significantly higher fetal/maternal lidocaine ratio than that reported after paracervical, pudendal, or epidural blocks.182 In contrast, 2-chloroprocaine was not detected in neonatal plasma at delivery.183 The complication associated with perineal infiltration is direct injection of local anesthetic into the fetal scalp, resulting in neonatal local anesthetic toxicity.184
Systemic analgesia
INTRAMUSCULAR/INTRAVENOUS OPIOID ADMINISTRATION
Systemic opioid analgesia is widely used, either as the sole analgesic agent or before administration of neuraxial labor analgesia. Systemic opioids provide some analgesia, particularly in early labor. Analgesia is incomplete for active labor. There is little scientific evidence that any one opioid is better than another. All have similar maternal and fetal side effects that are dose related. Maternal side effects include nausea, vomiting, delayed gastric emptying, dysphoria, and respiratory depression. All opioids cross the placenta. In utero, opioids may result in a slower fetal heart rate and decreased beat-to-beat variability.185 The likelihood of neonatal respiratory depression depends on the dose and timing of administration. Opioids may be administered by the subcutaneous, intramuscular, or intravenous route.
PATIENT-CONTROLLED INTRAVENOUS ANALGESIA
Patient-controlled intravenous analgesia (PCIA) has been used for labor analgesia.186 Although PCIA may result in higher patient satisfaction than nurse-administered opioid analgesia, studies have not shown reduced drug use or improved analgesia. Meperidine,187 nalbuphine,188 and fentanyl189 PCIA analgesia have been reported. No studies have adequately addressed optimal dosing, lockout periods, and continuous infusion-PCIA versus PCIA without a basal infusion. Recent reports document the use of a new opioid, remifentanil, PCIA in parturients.190, 191, 192, 193 Remifentanil has the theoretical advantage of rapid onset and offset (resulting in the ability to better titrate dose to pain, and in less neonatal depression).194
INHALATION ANALGESIA
The administration of inhalation analgesia for labor and vaginal delivery is unusual in the United States but is more common outside the United States. The only inhaled anesthetic agent still commonly used for labor analgesia is nitrous oxide; 50% nitrous oxide provides significant analgesia in approximately 50% of laboring parturients.195 The intermittent use of nitrous oxide during labor results in negligible fetal accumulation and does not depress neonatal respiration or neurobehavioral scores.196 Several studies suggest that the concomitant use of nitrous oxide and systemic opioids may increase the risk of maternal hypoxemia.197, 198 Safe administration of nitrous oxide necessitates an apparatus that limits the concentration of nitrous oxide, so a hypoxic mixture cannot be delivered. In addition, a mask or mouthpiece with a one-way valve should be used to limit pollution from unscavenged gases. Even so, environmental pollution from unscavenged gas may be significant.199
Volatile halogenated anesthetic agents have been used for labor analgesia. They are not routinely used for labor analgesia because of incomplete analgesia, concern about environmental pollution, and potential loss of maternal protective airway reflexes.200
OTHER FORMS OF ANALGESIA
Nonpharmacologic analgesic techniques include childbirth education, emotional support, massage, therapeutic use of hot and cold, and hydrotherapy. Techniques that require specialized training or equipment include biofeedback, transcutaneous electrical nerve stimulation (TENS), acupuncture or acupressure, intradermal water injections, and hypnosis.
Childbirth education is widely practiced. Studies of childbirth education tend to lack scientific rigor.201 Some investigators have found decreased use of pharmacologic analgesia, shorter labor, decreased incidence of operative deliveries, and fetal distress, whereas other investigators found no change in these variables.202
Emotional support often is provided by the parturient's husband or a friend. Several studies have documented salutary effects on the length of labor, analgesic requirements, and the incidence of operative deliveries when doulas (an unfamiliar support person not part of the medical establishment) are present during labor and delivery.203, 204 In randomized controlled trials, intradermal water injections compared to placebo or standard care have been shown to reduced severe low back pain during labor.205 Studies of biofeedback, TENS, acupuncture, and hypnosis do not show consistent benefits beyond routine childbirth education techniques.202
ANESTHESIA FOR CESAREAN DELIVERY
Neuraxial anesthesia
The most appropriate anesthetic technique for cesarean delivery depends on maternal, fetal, and obstetric factors. In the United States, neuraxial anesthesia has been used for over 80% of cesarean deliveries for many years.1 It is generally accepted that anesthetic-related maternal morbidity and mortaility are lower with neuraxial than general anesthesia. In a study of obstetric anesthesia mortality from 1979 to 1990, the anesthesia-related maternal mortality is 17 times higher for general anesthesia than for neuraxial anesthesia.35 A more recent analysis of data from 1997 to 2002 by the same authors suggests that the relative risk of general compared to neuraxial anesthesia may no longer be so high.206 However, most obstetric anesthesiologists recommend that neuraxial anesthesia should be administered whenever possible and that general anesthesia for cesarean delivery be used only when the benefits outweigh the risks.207, 208 Other advantages of neuraxial compared to general anesthesia include an awake mother able to participate in the childbirth (with the participation of a support person) and less drug transfer to the fetus/neonate.
SPINAL ANESTHESIA
Spinal anesthesia is indicated for most elective cesarean deliveries and for urgent cesarean deliveries in patients without pre-existing epidural labor analgesia. The advantages of spinal compared to epidural anesthesia include the following (Table 4):
- It is a technically easier block with a higher success rate;
- Onset of anesthesia is faster;
- The sensory block is more dense;
- There is no risk of systemic local anesthetic toxicity;
- There is minimal transfer of drug to the fetus.
Disadvantages include the following:
- It is not a continuous technique;
- Acute onset of sympathectomy may not be tolerated in select patients.
Table 4. Cesarean delivery anesthesia: epidural versus spinal
Epidural | Spinal | |
Onset | 15–30 min | 5–10 min |
Duration | Continuous | Single shot |
Success rate | Higher incidence of patchy, one-sided neuroblockade | |
Block quality | Less dense sensory block | More dense sensory block |
Less motor block | More motor block | |
Hypotension | Same incidence, slower onset | Same incidence, more rapid onset |
Risk of PDPH | Approximately 1% | Approximately 1% |
Risk of systemic local anesthetic toxicity | Inadvertent intravenous injection may cause systemic toxicity | Dose too small to cause systemic toxicity if inadvertently injected intravascular |
Risk of total spinal | Possible with inadvertent subarachnoid injection or “overdose” epidural injection | Less likely because of small drug dose |
Postcesarean delivery analgesia | Continuous or single-shot | Single-shot only |
Effects on the fetus | Greater drug exposure | Minimal drug exposure |
| Lower umbilical artery pH |
PDPH, postdural puncture headache.
With the advent of pencil-point spinal needles, the incidence of postdural puncture headache is approximately equal between spinal and epidural anesthesia.209 Meta-analysis comparing general, epidural, and spinal anesthesia suggests that spinal anesthesia is associated with a lower umbilicial artery pH and greater base deficit than general or epidural anesthesia (0.013–0.015 pH units).210 It is hypothesized that this finding may be due to the greater use of ephedrine to treat neuraxial anesthesia-induced hypotension with spinal anesthesia. Ephedrine crosses the placenta and may have a direct metabolic effect in the fetus (see later).211
Spinal anesthesia may be initiated in the sitting or lateral decubitus position. A high thoracic (T4) sensory level is necessary to block visceral stimulation. Local anesthetic choice depends on the desired duration of action; bupivacaine is the most commonly used local anesthetic. Opioids often are added to the local anesthetic. Lipid-soluble opioids (e.g., fentanyl and sufentanil) may decrease the incidence of intraoperative nausea and vomiting and increase the duration of anesthesia.212 Nausea and vomiting during cesarean delivery frequently are associated with peritoneal traction and exteriorization of the uterus. Opioids may help block this visceral response. Morphine often is added to the spinal anesthetic solution for postoperative analgesia (see later).
EPIDURAL ANESTHESIA
Epidural anesthesia frequently is used for parturients with indwelling epidural catheters placed for labor analgesia. The absolute dose of local anesthetic used for epidural anesthesia is 5–10 times greater than for spinal anesthesia; therefore, the epidural dose must be injected incrementally to rule out intrathecal or intravascular placement of the catheter. Intrathecal injection of an epidural dose results in total spinal anesthesia, and intravascular injection of an epidural dose results in systemic local anesthetic toxicity.
The advantage of epidural anesthesia is that the local anesthetic dose can be titrated to the desired sensory level. Theoretically, it should be easier to extend a “low” block or prevent a “high” block. However, the incidence of “patchy” blocks is increased with epidural compared with spinal anesthesia.
In some situations, the slow onset of epidural anesthesia may be a benefit. Epidural anesthesia results in a less-dense motor block than spinal anesthesia. This may be advantageous in patients with pulmonary disease who rely on abdominal and intercostal muscle tone for ventilation and clearing of pulmonary secretions. Block duration can be extended by reinjecting anesthetic through the epidural catheter. Finally, postoperative analgesia can be accomplished by continuous or incremental injection through the epidural catheter.
The choice of local anesthetic for epidural anesthesia depends on the desired onset time and duration of action. 2-chloroprocaine (3%) often is used for urgent cesarean deliveries because its onset time is quickest (5–10 minutes if epidural analgesia is already established). However, its duration of action is only 40–50 minutes. Lidocaine 2% with epinephrine 1:200,000 is the workhorse of epidural anesthesia. It has an intermediate-onset time (15 minutes, faster in presence of labor analgesia) and intermediate duration of action (75–100 minutes). Sodium bicarbonate may be added to lidocaine to speed the onset time.213 Bupivacaine has a longer onset time and greater risk of cardiac toxicity than lidocaine. Although bupivacaine also has a longer duration of action than lidocaine, the presence of the epidural catheter allows lidocaine to be reinjected should the procedure outlast the initial dose. Ropivacaine also has a long onset time and duration, and is associated with less cardiotoxicity than bupivacaine. Although local anesthetics cross the placenta during administration of epidural anesthesia, most studies show that there is no significant effect on neonatal neurobehavioral scores.135, 214
As with spinal anesthesia, opioids often are added to epidural local anesthetic solutions to enhance intraoperative anesthesia215, 216 and provide postoperative analgesia (see later).
COMBINED SPINAL-EPIDURAL ANESTHESIA
Combined spinal-epidural anesthesia can be used in several ways for cesarean delivery anesthesia. CSE anesthesia combines the advantages of spinal anesthesia (rapid onset of a dense block) with those of epidural anesthesia (ability to prolong the block). Anesthesia may be initiated with a standard spinal dose, followed by placement of the epidural catheter.217 The catheter is injected if the block needs to be prolonged. Alternatively, a “low” spinal dose can be injected, and the remaining initiation dose is injected through the epidural catheter. Proponents of this technique suggest that it is associated with a lower incidence of hypotension.218
GENERAL ANESTHESIA
General anesthesia for cesarean delivery is indicated any time neuraxial anesthesia is contraindicated, in the setting of dire fetal distress or failed neuraxial anesthesia. In contrast to neuraxial anesthesia, a prolonged incision to delivery time during general anesthesia may result in a greater incidence of neonatal acidosis,219 and lower 1-minute and 5-minute Apgar scores,220 presumably due to fetal exposure to general anesthetic agents. However, despite a higher incidence of newborn neonatal depression, there is no difference in ultimate neonatal outcome.220
The general anesthetic technique for cesarean delivery is straightforward. The airway is examined and aspiration prophylaxis is administered. The parturient is placed in left-lateral tilt and oxygen is administered by face mask in order to denitrogenate the lungs. If a difficult airway is anticipated, the parturient should be intubated awake before the induction of anesthesia. After preparations for surgery are completed, anesthesia is induced with a sedative-hypnotic agent, usually thiopental, followed by a neuromuscular blocking agent, usually succinylcholine. Because of the risk of aspiration, a rapid-sequence induction is performed (i.e., the patient is not ventilated until the endotracheal tube is in place). This prevents introduction of air into the stomach. Anesthesia is maintained with inhalation anesthetic (volatile anesthetic agent with or without nitrous oxide) until delivery of the neonate. After delivery, intravenous agents (e.g., opioids and benzodiazepines) usually are administered as the concentration of volatile agent is decreased. The volatile gases are smooth muscle relaxants and in high doses may contribute to uterine atony. The patient should have recovered protective airway reflexes before extubation of the trachea, as patients also are at risk of aspiration on emergence from anesthesia.
POSTCESAREAN DELIVERY ANALGESIA
The optimal postcesarean delivery analgesic regimen would provide profound analgesia, have no maternal or neonatal side effects, and have low cost. Multiple studies have shown superior analgesia with neuraxial versus systemic (intramuscular221, 222, 223, 224 or intravenous221, 222, 224, 225, 226, 227, 228) opioid administration. The total opioid dosage is significantly less, and mothers are significantly less sedated.222, 223, 224, 226, 228 In a study of nonobstetric, morbidly obese patients undergoing abdominal surgery, epidural morphine analgesia resulted in superior analgesia, less sedation, fewer pulmonary complications, and earlier return of bowel function compared with those of intramuscular morphine.229 In postcesarean delivery patients, a single-shot neuraxial opioid technique was associated with lower costs compared with PCIA.221
Neuraxial opioids may be administered by multiple techniques. Intrathecal morphine often is administered as a single-shot technique at initiation of spinal anesthesia. Epidural morphine may be administered as a single bolus after delivery. The duration of action of intrathecal or epidural morphine generally is 12–24 hours.138 Diamorphine has been used outside the United States.230, 231 Single bolus techniques have the advantage of being less cumbersome (the patient is not connected to a continuous infusion) and cheaper (no infusion pump is necessary). The disadvantage of single bolus techniques is that the dose is not titratable. Administering a higher dose may provide more satisfactory analgesia to all patients but at the cost of a higher incidence of undesirable side effects. In addition, the risk of late respiratory depression is greater with single bolus injection of hydrophilic opioids (see later).138
Alternatively, short-acting, lipophilic opioids (e.g., fentanyl and meperidine) may be administered by continuous epidural infusion232 or PCEA.223, 233
Side effects of neuraxial opioids include pruritus, nausea and vomiting, sedation, and respiratory depression. Most studies have found that pruritus occurs more commonly with neuraxial compared to systemic opioids.221, 222, 224, 228 The incidence of nausea and vomiting is similar between the two techniques.222, 223, 225, 226, 227, 228
Respiratory depression occurs after intramuscular, intravenous, and neuraxial opioid administration.234 Early respiratory depression (within 30–90 minutes of administration) occurs after the neuraxial administration of all opioids secondary to systemic absorption and transport of the drug via the circulatory system to the brain-stem respiratory centers.138 Late respiratory depression (6–10 hours after administration) occurs after a neuraxial bolus dose of morphine.235 Morphine is hydrophilic and circulates in the CSF to the brain stem. Risk factors for respiratory depression include advanced age and morbid obesity.138, 236 In 2009 the ASA published guidelines for the prevention, detection, and management of respiratory depression in patients who receive neuraxial opioids.237
Neuraxial opioid analgesia may be supplemented with systemic agents. The current trend is toward multimodal analgesic therapy. This allows the use of lower neuraxial opioid doses, resulting in fewer side effects. Analgesia can be supplemented with nonsteroidal anti-inflammatory agents (NSAIDs),238, 239, 240, 241, 242 acetaminophen,241 or low-dose PCIA-opioid administration. Recent publications have described the use of bilateral transversus abdominis plane (TAP) block for postcesarean delivery analgesia.243 The nerves that supply the anterior abdominal wall course through the neurofascial plane between the internal oblique and the transversus abdominis muscles. Injection of local anesthetic into this plan provides incisional analgesia after cesarean delivery.
Neuraxial analgesia is not practical or desirable for some patients. Studies comparing intramuscular to PCIA opioid administration after cesarean delivery found a comparable degree of analgesia.222, 224, 244 However, PCIA provided faster pain relief, fewer side effects, and greater patient satisfaction than intramuscular administration. In a study comparing PCIA meperidine to PCIA morphine after epidural morphine, PCIA meperidine was associated with neonatal neurobehavioral depression.245
PCIA can be administered with or without a continuous basal (background) infusion. In gynecologic surgery patients, a continuous background infusion did not improve pain relief or sleep but resulted in an increased total dosage and an increase in severity of side effects.246, 247 The inherent safety of the PCIA technique (patients only receive as much opioid as they need) is diminished if a continuous infusion is added.248 Therefore, a basal infusion is not recommended for patients recovering from cesarean delivery.249 Multimodal pain relief, including the use of NSAIDs and TAP block, should be encouraged, as this decreases reliance on systemic opioid analgesia and decreases the incidence of side effects.
ANESTHESIA FOR SPECIAL CIRCUMSTANCES
Considerations regarding anesthesia for special circumstances are shown in Table 5.
Table 5. Obstetric maternal complications and anesthetic considerations
Maternal Complication | Anesthetic Considerations |
Vaginal trial of labor after cesarean delivery | Low concentration of epidural local anesthetic in order not to mask the pain of uterine rupture |
Neuraxial analgesia-induced sympathetic blockade worsens hemorrhage-induced hypotension | |
Pre-eclampsia/eclampsia | Coagulopathy |
Airway edema causing a higher incidence of difficult airway | |
Interaction of magnesium with anesthetic techniques and drugs | |
Placenta previa | Neuraxial analgesia-induced sympathetic blockade worsens hemorrhage-induced hypotension |
Placental abruption | Possibility of DIC |
Neuraxial analgesia-induced sympathetic blockade worsens hemorrhage-induced hypotension | |
| Anticoagulation | Increased risk of spinal-epidural hematoma |
Postpartum hemorrhage | Neuraxial analgesia-induced sympathetic blockade worsens hemorrhage-induced hypotension |
Intrauterine fetal demise | DIC secondary to dead fetal tissue or placental abruption |
Chorioamnionitis | Possible increased risk of neuraxial infection |
DIC, disseminated intravascular coagulation;
Operative vaginal delivery
Dense sacral analgesia/anesthesia and perineal muscle relaxation facilitate operative delivery, particularly the application of forceps. This can be accomplished by extending segmental lumbar epidural analgesia or via spinal or CSE anesthesia, caudal epidural anesthesia, or a pudendal block.
Breech presentation
Neuraxial labor analgesia for planned breech vaginal delivery offers several benefits, including inhibition of early pushing, relaxation of the pelvic floor and perineum, and the option of extending anesthesia for emergency cesarean delivery. Early pushing may increase the risk of umbilical cord prolapse and delivery of a lower extremity before full cervical dilation. A relaxed pelvic floor at delivery facilitates delivery of the after-coming head. The challenge to the anesthesiologist is to provide enough first-stage analgesia to inhibit early pushing but minimize second-stage motor block to facilitate spontaneous delivery of the infant to the umbilicus and to rapidly extend the perineal block density at delivery to minimize head entrapment in the perineum and facilitate the application of forceps.
The fetal head may be entrapped in the perineum or more often in the cervix. Perineal skeletal muscle relaxation may be achieved with neuraxial anesthesia or by the administration of a neuromuscular blocking agent (usually succinylcholine) during the induction of general anesthesia. Cervical smooth muscle relaxation traditionally has been accomplished with the rapid sequence induction of general anesthesia, followed by the administration of a high concentration of a volatile anesthetic agent. More recently, intravenous or sublingual nitroglycerin has been used for uterine relaxation.250, 251, 252, 253
Multiple gestation
Neuraxial analgesia has several benefits in planned vaginal delivery with multiple gestation. Effective neuraxial analgesia facilitates internal podalic version and total breech extraction of twin B. Anesthesia can be extended for an emergency cesarean delivery, thus avoiding the risk of general anesthesia. In a retrospective study of 200 twin pregnancies, there was no difference in outcome between twins A and B (as assessed by Apgar score and umbilical cord blood gases) in women with epidural analgesia.254 In contrast, twin B has worse outcomes than twin A in women without epidural analgesia and the incidence of cesarean delivery for twin B was higher for women without analgesia. Similarly, in a retrospective study of 967 consecutive twin pregnancies eligible for vaginal delivery, the risk of delivering by combined vaginal (twin A) and cesarean (twin B) delivery was reduced if epidural labor analgesia was used during labor (RR 0.46; 95% CI 0.38–0.57).255
Vaginal trial of labor after cesarean delivery
In the past, there has been concern that neuraxial labor analgesia for vaginal trial of labor after cesarean delivery would mask the pain of uterine rupture. In addition, it was thought that the sympathectomy associated with neuraxial analgesia would prevent reflex hemodynamic compensation in the event of uterine rupture and hemorrhage. However, more recent data support the safety of neuraxial analgesia for vaginal trial of labor.
Several studies have found that abdominal pain or uterine tenderness has low sensitivity and specificity as a marker for uterine rupture or scar dehiscence.256, 257 There are reports of women with effective continuous epidural analgesia who acutely had breakthrough pain develop secondary to uterine rupture.258, 259, 260, 261 Most cases of lower uterine segment scar dehiscence do not result in severe maternal hemorrhage.262 Epidural analgesia did not affect the outcome of the trial of labor in several studies.263, 264 Finally, many women may agree to a trial of labor only if guaranteed effective analgesia.
In conclusion, most obstetricians and anesthesiologists and the ACOG265 agree that neuraxial analgesia is appropriate for vaginal trial of labor after cesarean delivery. Most anesthesiologists elect to maintain analgesia with dilute solutions of local anesthetic and opioid so that the pain of uterine rupture is not masked.
Pre-eclampsia/eclampsia
Several aspects of pre-eclampsia/eclampsia are of particular concern to anesthesiologists. These include (1) coagulation deficits that might preclude neuraxial anesthesia/analgesia, (2) airway edema, and (3) interaction of magnesium with anesthetic techniques and agents.
Approximately 10% of pre-eclampsia/eclamptic parturients have a platelet count of less than 100 x 109/L.266 Several investigators have described prolonged bleeding times in both thrombocytopenic and nonthrombocytopenic patients, presumably secondary to platelet dysfunction.267, 268 There are no data to support the use of a specific platelet count, above which neuraxial analgesia/anesthesia is “safe.” Epidural hematoma is a rare complication of neuraxial anesthesia. Almost all anesthesiologists initiate neuraxial anesthesia if the platelet count is above 100 x 109/L.269 A platelet count of 50 x 109/L seems to be the absolute lower limit practiced by most anesthesiologists. A review of systems and physical examination are both necessary to rule out a bleeding diathesis.
Also of concern to anesthesiologists is the parturient with a rapidly falling platelet count (e.g., patient with HELLP syndrome). In this circumstance, an epidural catheter may be placed several hours before the patient requires analgesia, before the platelet count is unacceptably low. This also allows an analgesic-free period to observe for symptoms of epidural hematoma. Finally, in parturients with falling platelet counts, a platelet count should be determined immediately before removal of the epidural catheter, as epidural hematoma formation has been described on epidural catheter removal.270
A small percentage of pre-eclampsia/eclamptic parturients have coagulation factor deficits. However, if the platelet count is greater than 100 x 109/L, determination of prothrombin time (PT) and activated partial thromboplastin time is not necessary.271
Most parturients with pre-eclampsia/eclampsia in the United States receive prophylactic magnesium sulfate therapy. Magnesium sulfate results in more profound maternal hypotension after the initiation of neuraxial anesthesia to the T10 level in gravid ewes.272 In addition, magnesium potentiates the action of neuromuscular blocking agents.273 Finally, magnesium blunts the response to vasoconstrictors.
Epidural analgesia may be the preferred method of labor analgesia for pre-eclampsia/eclamptic parturients for several reasons.274 First, pre-eclamptic parturients have an exaggerated hypertensive response to catecholamines and pain. This response is blunted by effective epidural analgesia, thus blood pressure control is facilitated.275 Second, epidural analgesia may improve intervillous blood flow.78 Finally, pre-eclamptic parturients are at increased risk of urgent cesarean delivery compared with normotensive parturients. The early initiation of epidural analgesia allows for epidural anesthesia (thus avoiding general anesthesia) should an urgent/emergent cesarean delivery be necessary.
General anesthesia for cesarean delivery in the pre-eclamptic parturient is associated with significant increases in systemic and pulmonary artery pressures275 and a decrease in intervillous blood flow.82 Compared to general anesthesia, neuraxial anesthesia is associated with a more stable hemodynamic profile and a decrease in the level of stress hormones. Epidural anesthesia also avoids transient neonatal depression associated with general anesthesia. Finally, epidural anesthesia avoids manipulation of the airway with the increased risk of difficult airway because of pharyngeal edema.276
In the past, controversy has existed as to whether spinal anesthesia is appropriate for the severely pre-eclamptic parturient. Opponents of spinal anesthesia argued that the rapid sympathectomy may result in profound hypotension in the volume-constricted, severely pre-eclamptic patient and that hypotension is less likely to be tolerated by a fetus with chronic uteroplacental insufficiency. In both a retrospective277 and prospective randomized trial,278 maternal and neonatal outcomes were not different between general and neuraxial anesthesia. Aya and colleagues compared maternal hemodynamic parameters during spinal anesthesia for cesarean delivery in healthy and severely preeclamptic women at term279 and preterm.280 Patients with preeclampsia had less severe spinal anesthesia-induced hypotension compared to the healthy cohort group. Finally, Dyer and colleagues randomized preeclamptic patients requiring urgent cesarean delivery for non-reassuring fetal status to general versus spinal anesthesia. The umbilical artery pH was lower in the spinal anesthesia group (7.20 vs. 7.23) and 1-min Apgar scores were lower in the general anesthesia group.281 However, there were no differences in the incidence of Apgar scores less than 7 at 1- or 5-min, or in requirements for resuscitation.
Placenta previa
The anesthetic implications of placenta previa revolve around the risk of maternal hemorrhage and neuraxial anesthesia induced sympathectomy. Hemorrhage was associated with significantly worse maternal hypotension, uterine blood flow, and fetal oxygenation in pregnant ewes with epidural anesthesia compared to control animals, unless the anesthetized ewes received prompt fluid resuscitation.282 Most obstetric anesthesiologists will consider neuraxial anesthesia for primary cesarean delivery in parturients who are not actively bleeding or hypotensive.283 The risk of placenta accreta increases markedly in patients with placenta previa and a previous uterine scar.284 Controversy exists regarding the appropriate type of anesthesia for patients at high risk of placenta accreta and cesarean hysterectomy. Arguments in favor of neuraxial anesthesia include several series in the literature that support the safety of this technique.285, 286, 287 In a series of 25 patients scheduled for elective cesarean hysterectomy, seven patients required the induction of general anesthesia after initial neuraxial anesthesia because of patient discomfort or inadequate operating conditions.285 Arguments against the use of neuraxial anesthesia include the potential for more severe maternal hypotension associated with neuraxial anesthesia, patient discomfort associated with intraperitoneal manipulation and traction, and patient discomfort associated with a prolonged surgical procedure. In addition, airway edema markedly worsens during volume resuscitation, thus increasing the risk of a difficult airway if urgent endotracheal intubation is necessary.288 In the circumstance of placenta previa and acute hemorrhage, general anesthesia is indicated.
Placental abruption
The anesthetic considerations in patients with placental abruption are similar to those with placenta previa. An additional consideration is the possible presence of disseminated intravascular coagulation (DIC). Neuraxial anesthesia is contraindicated in the presence of DIC. Patients who are hemodynamically stable without ongoing hemorrhage and without a coagulopathy are candidates for neuraxial analgesia/anesthesia. General anesthesia is indicated for acute hemorrhage or in the presence of DIC.
Anticoagulation
Neuraxial procedures are relatively contraindicated in the presence of drugs that alter the coagulation system because of the potential for bleeding into the neuraxis and the resulting compression of nerve tissue and risk of permanent neurologic injury. Because pulmonary embolism is a major cause of maternal mortality,289 it is increasingly common to encounter parturients who are anticoagulated with aspirin or heparin. National organizations have promolgated guidelines for the management of neuraxial anesthesia in the presence of anticoagulation.290, 291 In general, after the administration of low molecular weight heparin, the guidelines suggest a waiting period of 12–24 hours, depending on the dose. For these patients, the benefits of neuraxial analgesia/anesthesia must be weighed against the risk of spinal-epidural hematoma. The risk of hematoma appears to be greater after continuous epidural anesthesia than single-shot spinal anesthesia.270
Postpartum hemorrhage
The anesthetic considerations in postpartum hemorrhage are hemodynamic stability and maternal blood volume. For example, for repair of genital trauma, if the mother is hemodynamically stable and euvolemic, then extension of epidural analgesia or initiation of neuraxial anesthesia is appropriate. However, in the face of hypovolemia and hemodynamic instability, general anesthesia is the anesthetic of choice.
Intrauterine fetal demise
Intrauterine fetal demise can be associated with coagulation deficits for several reasons. Thromboplastin released by the dead fetus may lead to a consumptive coagulopathy, although this is unusual unless the fetus has been dead for more than a month.292 Approximately 15% of fetal deaths can be attributed to placental abruption, and abruption can be associated with DIC.293 Therefore, before initiating neuraxial analgesia in women with fetal demise, a disseminated coagulation profile may be indicated.
Prematurity
Although preterm infants probably are more vulnerable than the term infant to the effects of drugs used for analgesia and anesthesia, the general consensus is that direct drug effects are less important than preventing neonatal asphyxia and providing for an atraumatic delivery. In a secondary analysis of data prospectively gathered in France in 1997 (Etude éPIdémiologique sur les Petits Ages Gestationnels; EPIPAGE), spinal anesthesia was associated with a higher risk of neonatal death than general anesthesia (adjusted OR 1.7, 95% CI 1.1–2.6) in very preterm infants (27–33 weeks' gestation).294 Whether this represents a causal relationship is unclear and further study is warranted.
Chorioamnionitis
There has been much debate about the use of neuraxial analgesia/anesthesia in infected patients. The classic teaching has been that neuraxial anesthesia is relatively contraindicated in patients at risk for bacteremia. However, epidural abscess and meningitis associated with neuraxial anesthesia are rare events and bacteremia during labor is relatively common. Both epidural abscess and meningitis seem to occur less commonly in obstetric than in general surgery patients. Four reviews of more than 500,000 obstetric patients who received epidural analgesia/anesthesia found two cases of epidural abscess and no cases of meningitis.295, 296, 297, 298 In a retrospective survey study in Sweden, 29 cases of meningitis were idenfied after 1,260,000 spinal anesthetics for nonobstetric patients, and zero cases were idenfied among the 55,000 women who received spinal anesthesia for cesarean delivery.299 Similarly, 12 epidural abscesses were reported among 450,000 surgical patients, whereas only one abscess was identified among 200,000 labor epidural procedures.299 Given the frequency of fever and bacteremia (1% of laboring patients in one study300), it is likely that neuraxial procedures have been performed in many bacteremic obstetric patients without adverse outcome.
An additional issue is the difficulty in identifying which patients with chorioamnionitis are bacteremic. Fever and leukocytosis do not differentiate patients with positive and negative blood cultures.300, 301, 302 In an Escherichia coli bacteremic rat model, investigators found that administration of antibiotics before initiation of dural puncture protected the rat from the development of meningitis.303 The current general consensus of obstetric anesthesiologists is that neuraxial analgesia/anesthesia may be administered to parturients with chorioamnionitis after the systemic administration of antibiotics, unless they appear overtly septic.304
Anticipated difficult airway
Inability to intubate is a major cause of anesthesia morbidity and mortality. In the United Kingdom, the incidence of failed intubation was estimated at one in 2230 in the nonobstetric population6 but was one in 280–300 in the obstetric population.6, 305 In the United States between 1979 and 1990, the majority of anesthesia-related maternal mortality occurred during cesarean delivery and 49% were caused by a difficult airway.35 Overall, anesthesia-related mortality decreased in the triennium 1988 to 1990 compared to the triennium 1979–1982, presumably because the use of neuraxial anesthesia increased.
Several changes occur during pregnancy that may contribute to a difficult airway and its consequences. There is an increase in pharyngeal edema, made worse by pre-eclampsia and prolonged pushing efforts during the second stage. Weight gain also contributes to an increase in pharyngeal soft tissue. Enlarged breasts make the insertion of a standard laryngoscope handle difficult. Finally, a decrease in functional residual capacity (FRC) and an increase in oxygen consumption lead to more rapid arterial oxygen desaturation during apnea.20
Risk factors for a difficult airway include emergency surgery, obesity, short neck, missing or protruding maxillary incisors, receding mandible, facial edema, and a swollen tongue.306 Ideally, the potentially difficult airway should be identified as early as possible. The ACOG suggests that obstetricians be alert to the presence of risk factors and refer the patient for antenatal consultation by an anesthesiologist.307 Epidural analgesia is recommended for the laboring parturient with a potentially difficult airway, so that emergency general anesthesia can be avoided. If general anesthesia is necessary for a parturient with a recognized, potentially difficult airway, the airway should be secured before the induction of general anesthesia while the patient is awake and spontaneously breathing.
If failure to intubate occurs after the induction of anesthesia, a difficult airway algorithm should be followed.308 If mask ventilation is not possible, the ASA difficult airway algorithm should be followed; establishment of a surgical airway may be necessary. The most recent difficult airway algorithm has incorporated the laryngeal mask airway (LMA) as a rescue device.308 LMAs and other equipment (e.g., fiberoptic laryngoscopes) should be readily available in the obstetric operating room suite. If mask ventilation is possible but there is no fetal distress, the patient should be allowed to awaken, and other options for anesthesia and airway management should be implemented. If mask ventilation is possible but fetal distress is present, then delivery can proceed, albeit with an increased risk of maternal aspiration.
Malignant hyperthermia
Malignant hyperthermia (MH) is a hypermetabolic disease of skeletal muscle that is triggered in genetically susceptible individuals by exposure to volatile anesthetic agents or succinylcholine. It is life threatening if not recognized and treated appropriately. A history of malignant hyperthermia susceptibility in the parturient or her family should prompt an antenatal interview with an anesthesiologist. Epidural analgesia for labor decreases the likelihood that a patient with malignant hyperthermia susceptibility will require general anesthesia for emergency cesarean delivery. If a malignant hyperthermia crisis occurs during cesarean delivery, dantrolene should be administered as soon as possible, and steps should be taken to cool the patient and support ventilation and circulation.309 In the United States, expert consultant help can be obtained by calling the Malignant Hyperthermia Hotline at 1 - 800 - MHHYPER. A cart or kit with drugs and equipment to treat a MH crisis should be readily available in every location where general anesthesia is administered.
ANESTHESIA FOR OTHER PROCEDURES
External cephalic version
Elective external cephalic version (ECV) is an uncomfortable procedure. Many obstetricians prefer to perform the procedure with a small amount of maternal sedation, believing that more profound analgesia/anesthesia may increase the likelihood that excessive force will be used, resulting in increased morbidity and mortality. Several randomized controlled trials comparing neuraxial to no- or systemic analgesia on the success rate of ECV have found improved success in women randomized to neuraxial analgesia,310, 311, 312 while others have not.313, 314 A 2004 meta-analysis identified several negative studies that have only been published in abstract form, suggesting that publication bias may exist.315 An additional issue is that the studies are heterogeneous in terms of density of neuraxial blockade. The three randomized controlled trials in which the density of neuroblockade mimicked surgical anesthesia were all positive studies,310, 311, 312 whereas the two negative studies used neuraxial analgesia (density mimicked that used for labor analgesia).313, 314 In very small observational studies, parturients who elected to undergo neuraxial blockade for a second attempt at ECV after a first failed attempt had a higher successful rate than those who underwent a second attempt without neuraxial analgesia.316, 317 Currently, ACOG believes not enough information exists to support or oppose the use of neuraxial analgesia/anesthesia for external cephalic version.318 Further study is warranted.
Cervical cerclage
Neuraxial or general anesthesia is required for most women undergoing cervical cerclage. Neuraxial anesthesia is an excellent choice for women undergoing prophylactic cerclage. Some obstetricians prefer general anesthesia when the cervix is dilated or the membranes are bulging. They believe general anesthesia provides a decrease in intraabdominal pressure and uterine relaxation, thus decreasing the risk of membrane rupture and facilitating replacement of bulging membranes.319 However, coughing on the endotracheal tube during emergence from anesthesia, and vomiting, acutely increase intraabdominal pressure. Outcome differences between neuraxial and general anesthesia have not been well studied, although one retrospective study found no outcome differences between the two techniques.320
Manual exploration of the uterus and postpartum curettage
Manual exploration of the uterus and postpartum curettage often are facilitated by anesthesia. Neuraxial anesthesia may be extended or initiated in a hemodynamically, volume-resuscitated patient. Alternatively, nitrous oxide by face mask or small intravenous boluses of ketamine or opioid or both may provide sufficient analgesia. Uterine relaxation may be achieved by the administration of nitroglycerin321, 322 or general endotracheal anesthesia with a volatile anesthetic agent.
Hysterectomy
The anesthetic considerations for cesarean hysterectomy or postpartum hysterectomy are discussed in the Placenta previa section.
Postpartum tubal sterilization
Many anesthesiologists prefer to perform neuraxial anesthesia for immediate postpartum tubal sterilization because parturients still may be at increased risk for pulmonary aspiration in the immediate postpartum period. In particular, parturients who have received opioids for labor analgesia have delayed gastric emptying compared to parturients who do not receive opioids.28, 31, 32, 33 Both systemic28 and neuraxial opioids31, 32 delay gastric emptying compared with that of control subjects. Candidates for postpartum sterilization procedures should be hemodynamically stable and not have additional anesthetic risk factors (e.g., morbid obesity or difficult airway).
Either epidural or spinal anesthesia is appropriate. An epidural catheter placed for labor analgesia may be reinjected to provide surgical anesthesia. One study of epidural catheter reinjection suggests that the failure rate of epidural anesthesia increases as the delivery to surgery interval increases,323 whereas others have documented an 87–92% success rate regardless of time from delivery.324, 325 The neuraxial administration of a small dose of morphine improves postoperative analgesia after tubal ligation procedures.326, 327
Nonobstetric surgery during pregnancy
The maternal physiologic and anatomic changes that affect intrapartum anesthesia also affect the conduction of anesthesia for nonobstetric surgery during pregnancy. It often is difficult to determine whether fetal adverse outcomes are a result of maternal disease, the operation, or the anesthetic. Severe maternal hypoxemia and hypotension pose the greatest risk to the fetus. During the first trimester, fetuses are at risk for teratogenicity. No anesthetic agent is a proven teratogen.328 It is generally recommended that nonelective procedures be performed in the second trimester, as the risk of teratogencity is lower than during the first trimester and the risk of preterm labor is lower than in the third trimester.
COMPLICATIONS OF REGIONAL ANALGESIA/ANESTHESIA
Hypotension
Hypotension after neuraxial analgesia/anesthesia (defined as a 20% decrease in baseline blood pressure or a systolic blood pressure less than 100 mmHg) results from a decrease in cardiac output secondary to decreased preload resulting from increased venous capacitance after sympathetic blockade, and arteriolar vasodilation. Because uteroplacental perfusion is not autoregulated, uncorrected maternal hypotension may result in decreased uteroplacental perfusion and the development of fetal hypoxia and acidosis.329 Hypotension is more likely to occur in women who are not in labor at the time of induction of neuraxial anesthesia.330 Measures traditionally used to decrease the incidence and severity of maternal hypotension include left uterine displacement, or the lateral position, and prophylactic intravenous fluid administration.
Despite these measures, more than 70% of parturients develop hypotension after the induction of spinal anesthesia for elective cesarean delivery.331 Uncorrected hypotension results in fetal and neonatal acidosis.329 Small doses of a vasopressor usually readily correct the hypotension. Although laboratory studies of hypotension suggest that ephedrine maintains uterine blood flow better than pure α agonists,332 clinical studies suggest that phenylephrine is equally effective in treating maternal hypotension.333 Indeed, clinical studies comparing ephedrine (an indirectly acting mixed α- and β-sympathomimetic) to phenylephrine (a directly acting α-sympathomimetic) in healthy women undergoing scheduled ceserean delivery have found that umbilical artery pH is lower in women who receive ephedrine compared to phenylephrine.333 Ephedrine crosses the placenta in larger quantities than phenylephrine and is less readily metabolized, and in utero fetal exposure to ephedrine may result in direct metabolic effects.
Traditionally, a crystalloid "preload" (approximately 15–20 mL/kg) was administered before the initiation of neuraxial anesthesia in an attempt to "fill up the tank" and mitigate the relative hypovolemia that occurs after neuraxial-induced sympathectomy. Meta-analysis of randomized controlled trials of crystalloid preload versus no fluid bolus suggest that the practice of admininstering large volumes of fluid immediately before the initiation of analgesia may decrease the incidence of hypotension after high thoracic spinal anesthesia for cesarean delivery, but there is no evidence that a fluid bolus decreases the incidence of hypotension when administered before the initiation of neuraxial labor analgesia.334 Studies have shown the a colloid preload is superior to crystalloid preload.335 Administration of crystalloid coincident with the initation of anesthesia ("coload") has been shown to be superior to administering the fluid prior to the initiation of anesthesia.336 The administration of a crystalloid coload combined with a prophylactic phenylephrine infusion results in a very low incidence of hypotension.337
Fetal bradycardia
Fetal bradycardia occassionally occurs shortly after the initiation of labor neuraxial analgesia, even in the absence of hypotension. Clark and colleagues hypothesized that this may be due to an acute decrease in circulating epinephrine levels.338 Epinephrine, via its β-adrenergic receptor agonist activity, is a tocolytic, and the acute decrease in circulating epinephrine may lead to a temporary imbalance of uterine tocolytic/tocodynamic forces, resulting in uterine tachysystole, decreased uterine blood flow, and ultimately, fetal bradycardia. Some investigators have suggested that the incidence of fetal bradycardia is higher after neuraxial techniques that include the administration of intrathecal opioids.339 Nitroglycerine has been used to successfuly treat uterine tachysystole.340 Other measures include administration of an intravenous fluid bolus, discontinuing exogenous oxytocin administration, changing maternal position, and oxygen administration.
Postdural puncture headache
Postdural puncture headache (PDPH) is a complication of spinal or epidural anesthesia. It must be differentiated from other causes of postpartum headache. The pain often is localized to the frontal and occipital regions. It may radiate from the neck, and some patients report a stiff neck. Symptoms are markedly worse in the upright position and resolve, or almost completely resolve in the recumbent position. The severity of the headache ranges from mild to incapacitating and may be accompanied by nausea and vomiting. Photophobia, diplopia, difficulty in accommodation, hearing loss, hyperacusis, or tinnitus are symptoms of cranial nerve palsies caused by intracranial hypotension and stretching of the nerves.341 Seizures have been reported.342 Postdural puncture headaches commonly occur within 48 hours of dural puncture, and may last 7–10 days if left untreated. The incidence of PDPH is approximately 1% after dural puncture with a 25-gauge pencil-point spinal needle. The incidence of unintentonal dural puncture during initiation of epidural analgesia/anesthesia is approximately 1.5%.343 In a meta-analysis of unintentional dural puncture in obstetric patients, 52% of patients who suffered a dural puncture with an epidural needle went on to develop a PDPH.343
Treatment of PDPH depends on the severity of the symptoms. Mild headaches that do not interfere with activities of daily living can be treated conservatively. Conservative treatment includes assuming the horizontal position, preventing dehydration, and administering caffeine (intravenous344 or oral345). The standard treatment for moderate to severe headaches is an epidural blood patch. A single epidural blood patch results in complete cure in 33–68% of patients.346, 347, 348 The patch may be repeated if unsuccessful. The most common side effect of an epidural blood patch is backache.349
Maternal fever
A higher incidence of maternal fever has been observed in women who received epidural analgesia compared to those who do not. This observation was confirmed in a meta-analysis of randomized controlled trials of epidural versus systemic opioid analgesia.350 Recent investigation suggests that not all women with epidural analgesia have fever, but rather a subset of women whose temperature begins to increase immediately after initiation of analgesia.351 The mechanism is unclear, although evidence suggests it may be associated with markers of inflammation.352
Lieberman and coworkers353 reported a much higher incidence of neonatal sepsis workups in newborns delivered of mothers with epidural analgesia, although two thirds of the sepsis workups were performed in neonates without maternal fever. There is no evidence that epidural analgesia is associated with maternal or neonatal sepsis.
Back pain
Back pain is a common complaint both antepartum and postpartum. The most significant risk factors for postpartum back pain are antepartum backpain and inability to reduce weight after delivery.354 Randomized controlled trials of epidural compared to nonepidural analgesia failed to find a difference in the rate of postpartum back pain, even as long as several years after delivery.355, 356, 357
Inadvertent intravascular injection of local anesthetics
Inadvertent intravascular injection of local anesthetic agents may lead to systemic toxicity. At lower concentrations, local anesthetic toxicity involves the CNS. Higher concentrations result in cardiovascular toxicity. CNS toxicity is readily treatable with treatment of seizures and ventilatory support. However, serious maternal or neonatal sequelae may result if maternal hypoxemia is not treated expeditiously. Resuscitation from cardiac arrest secondary to local anesthetic toxicity may be particularly difficult in parturients.358 In addition to the standard treatment of cardiac arrest (ventricular dysrhythmia should be treated with amiodarone, not lidocaine), left uterine displacement is absolutely necessary to ensure adequate maternal circulation. Recent data suggest that administration of intravenous lipid emulsion may aid in resuscitation by binding of the fat to the local anesthetic.359 Prompt delivery of the fetus may be necessary.360
High or total spinal anesthesia
High or total spinal anesthesia may result from the inadvertent subarachnoid injection of a large “epidural” dose of local anesthetic, inadvertent subdural injection, or as a result of “overdosing” an epidural anesthetic. High neuraxial blockade results in hypotension, dyspnea, inability to speak, and loss of consciousness. Loss of consciousness and respiratory arrest are usually due to hypoperfusion of the brainstem (as opposed to brainstem anesthesia). Treatment is support of ventilation and circulation until the block dissipates.
Neurologic injury
SPACE-OCCUPYING LESIONS OF THE SPINAL CANAL
Epidural/spinal abscesses or hematomas are rare complications of neuraxial anesthesia. Hematomas are more likely to occur in patients with abnormal hemostasis.270 Although these hematomas are rare, timely diagnosis and treatment are essential to prevent permanent neurologic deficits. The risk of permAnent neurologic injury increases significantly if decompression is delayed beyond 6 hours after onset of symptoms.270 Signs and symptoms include backache, lower extremity sensory and motor deficits, bowel and bladder dysfunction. Fever and malaise are symptoms of epidural abscess. These signs and symptoms are not unusual after labor and delivery; therefore, a high index of suspicion must be maintained. Suspicion of spinal-epidural hematoma or abscess should prompt immediate imaging and neurosurgical consultation. Staphylococcus aureus is the most common causative organism of epidural abscess.361
Meningitis
Meningitis is a rare, but life-threatening, complication of neuraxial procedures in obstetric patients. The risk may be increased following procedures that involve dural puncture.361 The source of infection may be exogenous or endogenous, but unlike community acquired meningitis, usually involves streptococci of the viridans type.361 These organisms reside in the upper airway and vagina. Clusters of meningitis have been reported in the neurology, radiology, and anesthesiology literature.362, 363 These cases were thought to result from a break in sterile technique during the dural puncture. Symptoms of meningitis include fever, headache, photophobia, nausea, vomiting, and neck stiffness. Confusion, drowsiness, and a positive Kernig's sign may also be present. Diagnostic lumbar puncture is indicated; CSF should be cultured in broth because viridans streptococci are the most likely pathogens. Suspicion of meningitis should prompt immediate treatment with antibiotics, most often a third generation cephalosporin and vancomycin.
Peripheral nerve injury
Postpartum lower extremity nerve injury may be secondary to intrinsic maternal palsy, arising from the birth process, or secondary to neuraxial analgesia. Nerve injury secondary to epidural or spinal anesthesia is rare and often associated with paresthesia during initiation of the block.364, 365 A prospective study found a 1% incidence of intrinsic maternal palsy after labor and delivery.366 The most common injuries were to the lateral femoral cutaneous nerve (lateral thigh numbness) and femoral nerve (anterior thigh numbness and weakness of knee extension and hip flexion). Fortunately, both intrinsic maternal palsy and iatrogenic injuries usually resolve spontaneously.
SUMMARY
Childbirth is one of the most painful experiences a woman is likely to encounter in her lifetime. Fortunately, analgesic techniques exist that reduce or ameliorate labor pain. Neuraxial labor analgesia offers the most complete analgesia and is safe and reliable.
Anesthesia-related maternal morbidity and mortality have decreased steadily in recent years. This may be a result of the more widespread use of neuraxial analgesia and anesthesia in obstetric patients.
REFERENCES
Bucklin BA, Hawkins JL, Anderson JR et al: Obstetric anesthesia workforce survey: twenty-year update. Anesthesiology. 2005 Sep;103(3):645-53. |
|
Annibale DJ, Rosenfeld CR, Kamm KE: Alterations in vascular smooth muscle contractility during ovine pregnancy. Am J Physiol 256: H1282, 1989 |
|
Magness RR, Rosenfeld CR: Systemic and uterine responses to alpha-adrenergic stimulation in pregnant and nonpregnant ewes. Am J Obstet Gynecol 155: 897, 1986 |
|
Hohmann M, Keve TM, Osol G et al: Norepinephrine sensitivity of mesenteric veins in pregnant rates. J Physiol 259: R753, 1990 |
|
Assali NS, Prystowsky H: Studies on autonomic blockade. I. Comparison between the effects of tetraethylammonium chloride (TEAC) and high selective spinal anesthesia on blood pressure of normal and toxemic pregnancy. J Clin Invest 29: 1354, 1950 |
|
Samsoon GLT, Young JRB: Difficult tracheal intubation: A retrospective study. Anaesthesia 42: 487, 1987 |
|
Clark SL, Cotton DB, Pivarnik JM et al: Position change and central hemodynamic profile during normal third-trimester pregnancy and post partum. Am J Obstet Gynecol 164: 883, 1991 |
|
McLennan CE: Antecubital and femoral venous pressure in normal and toxemic pregnancy. Am J Obstet Gynecol 45: 568, 1943 |
|
Kerr MG, Scott DB, Samuel E: Studies of the inferior vena cava in late pregnancy. Br Med J 1: 532, 1964 |
|
Clark SL, Cotton DB, Lee W et al: Central hemodynamic assessment of normal term pregnancy. Am J Obstet Gynecol 161: 1439, 1989 |
|
Abitbol MM: Aortic compression by pregnant uterus. NY State J Med 76: 1470, 1976 |
|
Abitbol MM: Supine position in labor and associated fetal heart rate changes. Obstet Gynecol 65: 481, 1985 |
|
Huch A, Huch R, Schneider H et al: Continuous transcutaneous monitoring of fetal oxygen tension during labour. Br J Obstet Gynaecol 84 (Suppl 1): 1, 1977 |
|
Andrews PJD, Ackerman WE III Juneja MM: Aortocaval compression in the sitting and lateral positions during extradural catheter placement in the parturient. Can J Anaesth 40: 320, 1993 |
|
Spätling L, Fallenstein F, Huch A et al: The variability of cardiopulmonary adaptation to pregnancy at rest and during exercise. Br J Obstet Gynaecol 99 (Suppl 8): 1, 1992 |
|
Alaily AB, Carrol KB: Pulmonary ventilation in pregnancy. Br J Obstet Gynaecol 85: 518, 1978 |
|
Hägerdal M, Morgan CW, Sumner AE et al: Minute ventilation and oxygen consumption during labor with epidural analgesia. Anesthesiology 59: 425, 1983 |
|
Jouppila R, Hollmen A: The effect of segmental epidural analgesia on maternal and foetal acid-base balance, lactate, serum potassium and creatine phosphokinase during labour. Acta Anaesthesiol Scand 20: 259, 1976 |
|
McClelland SH, Bogod DG, Hardman JG: Pre-oxygenation and apnoea in pregnancy: changes during labour and with obstetricmorbidity in a computational simulation. Anaesthesia. 2009 Apr;64(4):371-7. |
|
Archer GW Jr, Marx GF: Arterial oxygen tension during apnoea in parturient women. Br J Anaesth 46: 358, 1974 |
|
Askrog VF, Smith TC, Eckenhoff JE: Changes in pulmonary ventilation during spinal anesthesia. Surg Gynecol Obstet 119: 563, 1964 |
|
Ulmsten U, Sundstrom G: Esophageal manometry in pregnant and nonpregnant women. Am J Obstet Gynecol 132: 260, 1978 |
|
VanThiel DH, Gavaler JS, Stremple J: Lower esophageal sphincter pressure in women using sequential oral contraception. Gastroenterology 71: 232, 1976 |
|
Whitehead EM, Smith M, Dean Y et al: An evaluation of gastric emptying times in pregnancy and the puerperium. Anaesthesia 48: 53, 1993 |
|
Macfie AG, Magides AD, Richmond MN et al: Gastric emptying in pregnancy. Br J Anaesth 67: 54, 1991 |
|
Wong CA, McCarthy RJ, Fitzgerald PC et al: Gastric emptying of water in obese pregnant women at term. Anesth Analg. 2007 Sep;105(3):751-5. |
|
Wong CA, Loffredi M, Ganchiff JN et al: Gastric emptying of water in term pregnancy. Anesthesiology. 2002 Jun;96(6):1395-400. |
|
O'Sullivan GM, Sutton AJ, Thompson SA et al: Noninvasive measurement of gastric emptying in obstetric patients. Anesth Analg 66: 505, 1987 |
|
Sandhar BK, Elliott RH, Windram I et al: Peripartum changes in gastric emptying. Anaesthesia 47: 196, 1992 |
|
Carp H, Jayaram A, Stoll M: Ultrasound examination of the stomach contents of parturients. Anesth Analg 74: 683, 1992 |
|
Ewah B, Yau K, King M et al: Effect of epidural opioids on gastric emptying in labour. Int J Obstet Anesth 2: 125, 1993 |
|
Wright PMC, Allen RW, Moore J et al: Gastric emptying during lumbar extradural analgesia in labor: Effect of fentanyl supplementation. Br J Anaesth 68: 248, 1992 |
|
Kelly MC, Carabine UA, Hill DA et al: A comparison of the effect of intrathecal and extradural fentanyl on gastric emptying in laboring women. Anesth Analg 85: 834, 1997 |
|
Nimmo WS, Wilson J, Prescott LF: Narcotic analgesic and delayed gastric emptying during labour. Lancet 1: 890, 1975 |
|
Hawkins JL, Koonin LM, Palmer SK et al: Anesthesia-related deaths during obstetric delivery in the United States, 1979-1990. Anesthesiology 86: 277, 1997 |
|
Mendelson CL: The aspiration of stomach contents into the lungs during obstetric anesthesia. Am J Obstet Gynecol 52: 191, 1946 |
|
Ollson GL, Hallen B, Hambraeus-Jonzon K: Aspiration during anaesthesia: A computer-aided study of 185,358 anaesthetics. Acta Anaesthesiol Scand 30: 84, 1986 |
|
Warner MA, Warner ME, Weber JG: Clinical significance of pulmonary aspiration during the perioperative period. Anesthesiology 78: 56, 1993 |
|
Gibbs CP, Rolbin SH, Norman P: Cause of prevention of maternal aspiration [letter]. Anesthesiology 61: 111, 1984 |
|
Gibbs CP, Schwartz DJ, Wynne JW et al: Antacid pulmonary aspiration in the dog. Anesthesiology 51: 380, 1979 |
|
Brock-Utne JG, Dow TG, Welman S et al: The effect of metoclopramide on the lower oesophageal sphincter in late pregnancy. Anaesth Intens Care 6: 26, 1978 |
|
Cohen SE, Jasson J, Talafre ML et al: Does metoclopramide decrease the volume of gastric contents in patients undergoing cesarean section? Anesthesiology 61: 604, 1984 |
|
Practice guidelines for obstetric anesthesia: an updated report by the AmericanSociety of Anesthesiologists Task Force on Obstetric Anesthesia. Anesthesiology. 2007 Apr;106(4):843-63. |
|
Scrutton MJ, Metcalfe GA, Lowy C et al: Eating in labour: A randomized controlled trail assessing the risks and benefits. Anaesthesia 54: 329, 1999 |
|
Simkin P: Stress, pain, and catecholamines in labor: Part 2. Stress associated withchildbirth events: a pilot survey of new mothers. Birth. 1986 Dec;13(4):234-40. |
|
ACOG Committee Opinion No. 441: Oral intake during labor. Obstet Gynecol. 2009 Sep;114(3):714. |
|
Yannone ME, McCurdy JR, Goldfien A: Plasma progesterone levels in normal pregnancy, labor, and the puerperium. II. Clinical data. Am J Obstet Gynecol 101: 1058, 1968 |
|
Iwasaki H, Collins JG, Saito Y et al: Naloxone-sensitive, pregnancy-induced changes in behavioral responses to colorectal distention: Pregnancy-induced analgesia to visceral stimulation. Anesthesiology 74: 927, 1991 |
|
Dalby PL, Ramanathan S, Rudy TE et al: Plasma and saliva substance P levels: The effects of acute pain in pregnant and non-pregnant women. Pain 69: 263, 1997 |
|
Datta S, Hurley RJ, Naulty JS et al: Plasma and cerebrospinal fluid progesterone concentrations in pregnant and nonpregnant women. Anesth Analg 65: 950, 1986 |
|
Kaneko M, Kirhara Y, Kosaka Y: Pregnancy enhances the antinociceptive effects of extradural lignocaine in the rat. Br J Anaesth 72: 657, 1994 |
|
Popitz-Bergez FA, Leeson S, Thalhammer JG et al: Intraneural lidocaine uptake compared with analgesic differences between pregnant and nonpregnant rats. Reg Anesth 22: 363, 1997 |
|
Bromage PR: Spread of analgesic solutions in the epidural space and their site of action: A statistical study. Br J Anaesth 34: 161, 1962 |
|
Butterworth JF IV, Walker FO, Lysak SZ: Pregnancy increases median nerve susceptibility of lidocaine. Anesthesiology 72: 962, 1990 |
|
Lauretti GR: Mechanisms of labor pain. In Norris MC (ed): Obstetric Anesthesia, pp 235–249. 2nd ed. Philadelphia, Lippincott Williams & Wilkins, 1999 |
|
Hirabayashi Y, Shimizu R, Fukada H et al: Soft tissue anatomy within the vertebral canal in pregnant women. Br J Anaesth 77: 153, 1996 |
|
Hogan QH, Prost R, Kulier A et al: Magnetic resonance imaging of cerebrospinal fluid volume and the influence of body habitus and abdominal pressure. Anesthesiology 84: 1341, 1996 |
|
Carpenter RL, Hogan QH, Liu SS et al: Lumbosacral cerebrospinal fluid volume is the primary determinant of sensory block extent and duration during spinal anesthesia. Anesthesiology 89: 24, 1998 |
|
Richardson MG, Wissler RN: Density of lumbar cerebrospinal fluid in pregnant and nonpregnant humans. Anesthesiology. 1996 Aug;85(2):326-30. |
|
Abouleish EI: Postpartum tubal ligation requires more bupivacaine for spinal anesthesia than does cesarean section. Anesth Analg 65: 897, 1986 |
|
Morishima HO, Finster M, Arthur R et al: Pregnancy does not alter lidocaine toxicity. Am J Obstet Gynecol 162: 320, 1990 |
|
Santos AC, Arthur GR, Pedersen H et al: Systemic toxicity of ropivacaine during ovine pregnancy. Anesthesiology 75: 137, 1991 |
|
Morishima HO, Pedersen H, Finster M et al: Bupivacaine toxicity in pregnant and nonpregnant ewes. Anesthesiology 63: 134, 1985 |
|
Santos AC, Arthur GR, Wlody D: Comparative systemic toxicity of ropivacaine and bupivacaine in nonpregnant and pregnant ewes. Anesthesiology 82: 734, 1995 |
|
Palahnuik RJ, Shnider SM, Eger EI II: Pregnancy decreases the requirement for inhaled anesthetic agents. Anesthesiology 41: 82, 1974 |
|
Gin T, Mainland P, Chan MTV et al: Decreased thiopental requirements in early pregnancy. Anesthesiology 86: 73, 1997 |
|
Morgan DJ, Blackman GL, Paull JD et al: Pharmacokinetics and plasma binding of thiopental. II. Studies at cesarean section. Anesthesiology 54: 474, 1981 |
|
Wood M, Wood AJJ: Changes in plasma drug binding and alpha1-acid glycoprotein in mother and newborn infant. Clin Pharmacol Ther 29: 522, 1981 |
|
Fragneto RY, Bader AM, Rosinia F et al: Measurements of protein binding of lidocaine throughout pregnancy. Anesth Analg 79: 295, 1994 |
|
Cummings AJ: A survey of pharmacokinetic data from pregnant women. Clin Pharmacokinet 8: 344, 1983 |
|
Ngan Kee WD. Uteroplacental blood flow. In Chestnut DH, Polley LS, Tsen LC, Wong CA (eds): Obstetric Anesthesia: Principles and Practice, 4th ed. pp. 37-53. Philadelphia: Elsevier Mosby 2009. |
|
Shnider SM, Wright RG, Levinson G et al: Uterine blood flow and plasma norepinephrine changes during maternal stress in the pregnant ewe. Anesthesiology 50: 524, 1979 |
|
Shnider SM, Abboud T, Artal R et al: Maternal catecholamines decrease during labor after lumbar epidural analgesia. Am J Obstet Gynecol 147: 13, 1983 |
|
Abboud TK, Artal R, Henriksen EH et al: Effect of spinal anesthesia on maternal circulating catecholamines. Am J Obstet Gynecol 142: 252, 1982 |
|
Lederman RP, Lederman E, Work B et al: Anxiety and epinephrine in multiparous labor: Relationship to duration of labor and fetal heart rate pattern. Am J Obstet Gynecol 153: 870, 1985 |
|
Motoyama EK, Fuchigami T, Gotto H et al: Response of fetal placental vascular bed to changes in pCO2 in sheep. In Longo LD, Reneau DR (eds): Fetal and Newborn Cardiovascular Physiology, pp 33–46. New York, Garland STPM Press, 1978 |
|
Hollmen A, Jouppila R, Jouppila P et al: Effect of extradural analgesia using bupivacaine and 2-chloroprocaine on intervillous blood flow during normal labor. Br J Anaesth 54: 837, 1982 |
|
Jouppila P, Jouppila R, Hollmen A et al: Lumbar epidural analgesia to improve intervillous blood flow during labor in severe preeclampsia. Obstet Gynecol 59: 158, 1982 |
|
Hood DD, Dewan DM, James III FM: Maternal and fetal effects of epinephrine in gravid ewes. Anesthesiology 64: 610, 1986 |
|
Alahuhta S, Rasanen J, Jouppila R et al: Effects of extradural bupivacaine with adrenaline for Cesarean section on uteroplacental and fetal circulation. Br J Anaesth 67: 678, 1991 |
|
Chestnut DH, Weiner CP, Herrig JE: The effect of intravenously administered 2-chloroprocaine upon uterine artery blood flow velocity in gravid guinea pigs. Anesthesiology 70: 305, 1989 |
|
Jouppila P, Kuikka J, Jouppila R et al: Effect of induction of general anesthesia for cesarean section on intervillous blood flow. Acta Obstet Gynecol Scand 58: 249, 1979 |
|
Levinson G, Shnider SM, deLorimier AA et al: Effects of maternal hyperventilation on uterine blood flow and fetal oxygenation and acid-base status. Anesthesiology 40: 340, 1974 |
|
Morishima HO, Daniel SS, Adamson Jr K et al: Effects of positive pressure ventilation of the mother upon the acid-base state of the fetus. Am J Obstet Gynecol 93: 269, 1965 |
|
Zakowski MI, Herman NL. The placenta: anatomy, physiology, and transfer of drugs. In Chestnut DH, Polley LS, Tsen LC, Wong CA (eds): Obstetric Anesthesia: Principles and Practice, 4th ed. pp. 55-72. Philadelphia: Elsevier Mosby 2009. |
|
Melzack R, Taezner P, Feldman P et al: Labour is still painful after prepared childbirth training. Can Med Assoc J 125: 357, 1981 |
|
Bonica JJ: The nature of pain in parturition. Clin Obstet Gynecol 2: 499, 1975 |
|
Capogna G, Celleno D, Lyons G et al: Minimum local analgesia concentration of extradural bupivacaine increases with progression of labor. Br J Anaesth 80: 11, 1998 |
|
Viscomi CM, Rathmell JP, Pace NL: Duration of intrathecal labor analgesia: Early versus advanced labor. Anesth Analg 84: 1108, 1997 |
|
Paech MJ: The King Edward Memorial Hospital 1,000 mother survey of methods of pain relief in labour. Anaesth Intensive Care 19: 393, 1991 |
|
Aarnoudse JG, Oeseburg G, Kwat A et al: Influence of variations in pH and pCO2 on scalp tissue oxygen tension and carotid arterial oxygen tension in the fetal lamb. Biol Neonate 40: 252– 263, 1981 |
|
Peabody JH: Transcutaneous oxygen measurement to evaluate drug effects. Clin Perinatol 6: 109, 1979 |
|
Sangoul F, Fox GS, Houle GL: Effect of regional analgesia on maternal oxygen consumption during the first stage of labor. Am J Obstet Gynecol 212: 1080, 1975 |
|
Griffen RP, Reynolds F: Maternal hypoxemia during labour and delivery: The influence of analgesia and effect on neonatal outcome. Anaesthesia 50: 151, 1995 |
|
Huch R: Maternal hyperventilation and the fetus. J Perinatol Med 14: 3, 1986 |
|
Ueland K, Hansen JM: Maternal cardiovascular dynamics. III. Labor and delivery under local and caudal analgesia. Am J Obstet Gynecol 103: 8, 1969 |
|
Ramos-Santos E, Devoe LD, Wakefield ML et al: The effects of epidural anesthesia on the Doppler velocimetry of umbilical and uterine arteries in normal and hypertensive patients during active term labor. Obstet Gynecol 77: 20, 1991 |
|
Thomas TA, Fletcher JE, Hill RG: Influence of medication, pain, and progress in labour on plasma-endorphin-like immunoreactivity. Br J Anaesth 54: 401, 1982 |
|
Jouppila R, Puolakka J, Kaupila A et al: Maternal and umbilical cord plasma noradrenaline concentration during labour with and without segmental extradural analgesia and during Cesarean section. Br J Anaesth 56: 251, 1984 |
|
Abboud TK, Yanagi T, Artal R et al: Effect of epidural analgesia during labor on fetal plasma catecholamine release. Reg Anesth 10: 170, 1985 |
|
Anderson HT, Skrede S: Obstetrical analgesia assessed by free fatty acid metabolism. Acta Anaesthesiol Scand 19: 245, 1975 |
|
Pearson JF, Davies P: The effect of continuous lumbar epidural analgesia on maternal acid base balance and arterial lactate concentrations during the second stage of labour. J Obstet Gynaecol Br Commonw 80: 225, 1973 |
|
Pearson JF, Davies P: The effect of continuous lumbar epidural analgesia on the acid-base status of maternal arterial blood during the first stage of labour. J Obstet Gynaecol Br Commonw 80: 218, 1973 |
|
Thalme B, Raabe N, Belfrage P: Lumbar epidural analgesia in labour. II. Effects on glucose, lactate, sodium chloride, total protein, haematocrit and haemoglobin in maternal, fetal and neonatal blood. Acta Obstet Gynecol Scand 53: 113, 1974 |
|
Climie CR: The place of continuous lumbar epidural analgesia in the management of abnormally prolonged labour. Med J Aust 2: 447, 1964 |
|
Moir DD, Willocks J: Management of incoordinate uterine action under continuous epidural analgesia. Br Med J 3: 396, 1967 |
|
Wuitchik M, Bakal D, Lipshitz J: The clinical significance of pain and cognitive activity in latent labor. Obstet Gynecol 73: 35, 1989 |
|
Socol ML, Garcia PM, Peaceman AM et al: Reducing cesarean births at a primarily private university hospital. Am J Obstet Gynecol 168: 1748, 1993 |
|
Neuhoff D, Burke MS, Porreco RP: Cesarean birth for failed progress in labor. Obstet Gynecol 73: 915, 1989 |
|
Halpern SH, Leighton BL: Epidural analgesia and the progress of labor. In Halpern SH, Douglas MJ, editors. Evidence-Based Obstetric Anesthesia. Oxford, UK, Blackwell, 2005:10-22 |
|
Zhang J, Yancey MK, Klebanoff MA et al: Does epidural analgesia prolong labor and increase risk of cesarean delivery? Anatural experiment. Am J Obstet Gynecol. 2001 Jul;185(1):128-34. |
|
Segal S, Su M, Gilbert P: The effect of a rapid change in availability of epidural analgesia on the Am J Obstet Gynecol. 2000 Oct;183(4):974-8. |
|
Wong CA, Scavone BM, Peaceman AM et al: The risk of cesarean delivery with neuraxial analgesia given early versus late inlabor. N Engl J Med. 2005 Feb 17;352(7):655-65. |
|
Ohel G, Gonen R, Vaida S et al: Early versus late initiation of epidural analgesia in labor: does it increase therisk of cesarean section? A randomized trial. Am J Obstet Gynecol. 2006 Mar;194(3):600-5. |
|
Wong CA, McCarthy RJ, Sullivan JT et al: Early compared with late neuraxial analgesia in nulliparous labor induction: arandomized controlled trial. Obstet Gynecol. 2009 May;113(5):1066-74. |
|
Wang F, Shen X, Guo X et al: Epidural analgesia in the latent phase of labor and the risk of cesarean delivery: a five-year randomized controlled trial. Anesthesiology. 2009 Sep 7. [Epub ahead of print] |
|
Marucci M, Cinnella G, Perchiazzi G et al: Patient-requested neuraxial analgesia for labor: impact on rates of cesarean andinstrumental vaginal delivery. Anesthesiology. 2007 May;106(5):1035-45. |
|
Thorp JA, Hu DH, Albin RM et al: The effect of intrapartum epidural analgesia on nulliparous labor: A randomized, controlled, prospective trial. Am J Obstet Gynecol 169: 851, 1993 |
|
Bofill JA, Vincent RD, Ross EL et al: Nulliparous active labor, epidural analgesia, and cesarean delivery for dystocia. Am J Obstet Gynecol 177: 1465, 1997 |
|
Chestnut DH, McGrath JM, Vincent RD et al: Does early administration of epidural analgesia affect obstetric outcome in nulliparous women who are in spontaneous labor. Anesthesiology 80: 1201, 1994 |
|
Chestnut DH, Vincent RD, McGrath JM et al: Does early administration of epidural analgesia affect obstetric outcome in nulliparous women who are receiving intravenous oxytocin? Anesthesiology 90: 1193, 1994 |
|
Sharma SK, Sidawi JE, Ramin SM et al: Cesarean delivery: A randomized trial of epidural versus patient-controlled meperidine analgesia during labor. Anesthesiology 87: 487, 1997 |
|
Zamora JE, Rosaeg OP, Lindsay MP et al: Haemodynamic consequences and uterine contractions following 0.5 or 1.0 litre crystalloid infusion before obstetric epidural analgesia. Can J Anaesth 43: 347, 1996 |
|
Cheek TG, Samuels P, Tobin M et al: Rapid intravenous saline infusion decreases uterine activity in labor, epidural analgesia does not [abstract]. Anesthesiology 71: A884, 1989 |
|
Sharma SK, McIntire DD, Wiley J et al: Labor analgesia and cesarean delivery: an individual patient meta-analysis ofnulliparous women. Anesthesiology. 2004 Jan;100(1):142-8; discussion 6A. |
|
COMET Study Group: Effect of low-dose mobile versus traditional epidural techniques on mode ofdelivery: a randomised controlled trial. Lancet. 2001 Jul 7;358(9275):19-23. |
|
Nageotte MP, Larson D, Rumney PJ et al: Epidural analgesia compared with combined spinal-epidural analgesia during laborin nulliparous women. N Engl J Med. 1997 Dec 11;337(24):1715-9. |
|
Roberts CL, Torvaldsen S, Cameron CA et al: Delayed versus early pushing in women with epidural analgesia: a systematic BJOG. 2004 Dec;111(12):1333-40. |
|
ACOG Practice Bulletin Number 49, December 2003: Dystocia and augmentation oflabor. Obstet Gynecol. 2003 Dec;102(6):1445-54. |
|
Philipsen T, Jensen N-H: Epidural block or parenteral pethidine as analgesic in labour: A randomized study concerning progress in labour and instrumental deliveries. Eur J Obstet Gynecol Reprod Biol 30: 27, 1989 |
|
Ramin SM, Gambling DR, Lucas MJ et al: Randomized trial of epidural versus intravenous analgesia during labor. Obstet Gynecol 86: 783, 1995 |
|
Belfrage P, Berlin A, Raabe N et al: Lumbar epidural analgesia with bupivacaine in labor. Drug concentration in maternal and neonatal blood at birth and during the first day of life. Am J Obstet Gynecol 123: 839, 1975 |
|
Kennedy RL, Bell JU, Miller RP et al: Uptake and distribution of lidocaine in fetal lambs. Anesthesiology 72: 483, 1990 |
|
Kuhnert BR, Harrison MJ, Linn PL et al: Effects of maternal epidural anesthesia on neonatal behavior. Anesth Analg 63: 301, 1984 |
|
Abboud TK, Kim KC, Noueihed R: Epidural bupivacaine, chloroprocaine, or lidocaine for cesarean section: Maternal and neonatal effects. Anesth Analg 62: 914, 1983 |
|
Kuhnert BR, Kuhnert PM, Philipson EH et al: The half-life of 2-chloroprocaine. Anesth Analg 65: 273, 1986 |
|
Vercauteren M, Meert TF: Isobolographic analysis of the interaction between epidural sufentanil and bupivacaine in rats. Pharmacol Biochem Behav 58: 237, 1997 |
|
Cousins MJ, Mather LE: Intrathecal and epidural administration of opioids. Anesthesiology 61: 276, 1984 |
|
Vertommen JD, Vandermeulen E, Van Aken H: The effects of the addition of sufentanil to 0.125% bupivacaine on the quality of analgesia during labor and on the incidence of instrumental deliveries. Anesthesiology 74: 809, 1991 |
|
Justins DM, Francis D, Houlton PG et al: A controlled trial of extradural fentanyl in labour. Br J Anaesth 54: 409, 1982 |
|
Steinberg RB, Powell G, Hu XH et al: Epidural sufentanil for analgesia for labor and delivery. Reg Anesth 14: 225, 1989 |
|
Bader AM, Fragneto R, Terui K et al: Maternal and neonatal fentanyl and bupivacaine concentration after epidural infusion during labor. Anesth Analg 81: 829, 1995 |
|
Porter JS, Bonello E, Reynolds F: The effect of epidural opioids on maternal oxygenation during labour and delivery. Anaesthesia 51: 899, 1996 |
|
Sinatra RS, Sevarino FB, Chung JH et al: Comparison of epidurally administered sufentanil, morphine, and sufentanil-morphine combination for postoperative analgesia. Anesth Analg 72: 522, 1991 |
|
Bogod DG, Rosen M, Rees GA: Extradural infusion of 0.125% bupivacaine at 10 mL/hr to women during labor. Br J Anaesth 59: 325, 1987 |
|
Eddleston JM, Maresh M, Horsman EL et al: Comparison of the maternal and fetal effects associated with intermittent or continuous infusion of extradural analgesia. Br J Anaesth 69: 154, 1992 |
|
Ewen A, Mcleod DD, Mcleod DM: Continuous infusion epidural analgesia in obstetrics: A comparison of 0.08% and 0.25% bupivacaine. Anaesthesia 41: 143, 1986 |
|
Lamont RF, Pinney D, Rodgers P et al: Continuous versus intermittent epidural analgesia. Anaesthesia 44: 893, 1989 |
|
Li DF, Rees GAD, Rosen M: Continuous extradural infusion of 0.0625% or 0.125% bupivacaine for pain relief in primigravid labour. Br J Anaesth 57: 264, 1985 |
|
Hicks JA, Jenkins JG, Newton MC et al: Continuous epidural infusion of 0.075 % bupivacaine for pain relief in labour. Anaesthesia 43: 289, 1988 |
|
Smedstad KG, Morison DH: A comparative study of continuous and intermittent epidural analgesia for labour and delivery. Can J Anaesth 35: 234, 1988 |
|
Gambling DR, McMorland GJ, Yu P et al: Comparison of patient-controlled epidural analgesia and conventional intermittent “top-up” injections during labor. Anesth Analg 70: 256, 1990 |
|
Curry PD, Pacsoo C, Heap DG: Patient-controlled epidural analgesia in obstetric anaesthetic practice. Pain 57: 125, 1994 |
|
Ferrante FM, Rosinia FA, Gordon C et al: The role of continuous background infusions in patient-controlled epidural analgesia for labor and delivery. Anesth Analg 79: 80, 1994 |
|
Gambling DR, Yu P, Cole C: A comparative study of patient controlled epidural analgesia (PCEA) and continuous infusion epidural analgesia (CIEA) during labour. Can J Anaesth 35: 249, 1988 |
|
D'Angelo RD, Anderson MT, Phillip J et al: Intrathecal sufentanil compared to epidural bupivacaine for labor analgesia. Anesthesiology 80: 1209, 1994 |
|
Dunn SM, Connelly NR, Steinberg RB et al: Intrathecal sufentanil versus epidural lidocaine with epinephrine and sufentanil for early labor analgesia. Anesth Analg 87: 331, 1998 |
|
Norris MC, Grieco WM, Borkowski M et al: Complications of labor analgesia: Epidural versus combined spinal epidural techniques. Anesth Analg 79: 529, 1994 |
|
Hughes SC: Respiratory depression following intraspinal narcotics: expect it! Int J Obstet Anesth. 1997 Jul;6(3):145-6. |
|
Herman NL, Choi KC, Affleck PJ et al: Analgesia, pruritus, and ventilation exhibit a dose-response relationship in parturients receiving intrathecal fentanyl during labor. Anesth Analg. 1999 Aug;89(2):378-83. |
|
Abboud TK, Sheik-ol-Eslam A, Yanagi T et al: Safety of efficacy of epinephrine added to bupivacaine for lumbar epidural analgesia in obstetrics. Anesth Analg 64: 585, 1985 |
|
Eisenach JC, Grice SC, Dewan DM: Epinephrine enhances analgesia produced by epidural bupivacaine during labor. Anesth Analg 66: 447, 1987 |
|
Campbell DC, Banner R, Crone LA et al: Addition of epinephrine to intrathecal bupivacaine and sufentanil for ambulatory labor analgesia. Anesthesiology 86: 525, 1997 |
|
Eisenach JC, DeKock M, Klimscha W: Alpha(2)-adrenergic agonists for regional anesthesia. A clinical review of clonidine. Anesthesiology 85: 655, 1996 |
|
Roelants F: The use of neuraxial adjuvant drugs (neostigmine, clonidine) in obstetrics. Curr Opin Anaesthesiol. 2006 Jun;19(3):233-7. |
|
Teramo K: Effects of obstetrical paracervical blockade on the fetus. Acta Obstet Gynecol Scand 16 (Suppl): 1, 1971 |
|
Weiss RR, Halevy S, Almonte RO et al: Comparison of lidocaine and 2-chloroprocaine in paracervical block: Clinical effects and drug concentrations in mother and child. Anesth Analg 62: 168, 1983 |
|
LeFevre ML: Fetal heart rate pattern and postparacervical fetal bradycardia. Obstet Gynecol 64: 343, 1984 |
|
Gaylord TG, Pearson JW: Neuropathy following paracervical block in the obstetric patient. Obstet Gynecol 60: 521, 1982 |
|
Svancarek W, Chirino O, Schaefer G et al: Retropsoas and subgluteal abscesses following paracervical and pudendal anesthesia. JAMA 237: 892, 1977 |
|
O'Meara OP, Brazie JV: Neonatal intoxication after paracervical block [letter]. N Engl J Med 278: 1127, 1968 |
|
Chestnut DH. Alternative regional anesthetic techniques: Paracervical block, lumbar sympathetic block, pudendal nerve block, and perineal infiltration. In Chestnut DH, Polley LS, Tsen LC, Wong CA (eds): Obstetric Anesthesia: Principles and Practice, 4th ed. pp. 493-503. Philadelphia: Elsevier Mosby 2009. |
|
Morishma HO, Covino BG, Yeh MN et al: Bradycardia in the fetal baboon following paracervical block anesthesia. Am J Obstet Gynecol 140: 775, 1981 |
|
Fishburne JI, Greiss FC, Hopkinson R et al: Responses of the gravid uterine vasculature to arterial levels of local anesthetic agents. Am J Obstet Gynecol 133: 753, 1979 |
|
Suelto MD, Shaw DB: Labor analgesia with paravertebral lumbar sympathetic block. Reg Anesth Pain Med 24: 179, 1999 |
|
Hunter CA: Uterine motility studies during labor: Observations on bilateral sympathetic nerve block in the normal and abnormal first stage of labor. Am J Obstet Gynecol 85: 681, 1963 |
|
Leighton BL, Halpern SH, Wilson DB: Lumbar sympathetic blocks speed early and second stage induced labor in nulliparous women. Anesthesiology 90: 1039, 1999 |
|
Scudamore JH, Yates MJ: Pudendal block—a misnomer? Lancet 1: 23, 1966 |
|
Zador G, Lindmark G, Nilsson BA: Pudendal block in normal vaginal deliveries. Acta Obstet Gynecol Suppl 1974:34:51, 1974 |
|
Kurzel RB, Au AH, Rooholamini SA: Retroperitoneal hematoma as a complication of pudendal block: diagnosis made by computed tomography. West J Med 164: 523, 1996 |
|
Chase D, Brady JP: Ventricular tachycardia in a neonate with mepivacaine toxicity. J Pediatr 90: 127, 1977 |
|
Philipson EH, Kuhnert BR, Syracuse CD: Maternal, fetal, and neonatal lidocaine levels following local perineal infiltration. Am J Obstet Gynecol 149: 403, 1984 |
|
Philipson EH, Kuhnert BR, Syracuse CD: 2-Chloroprocaine for local perineal infiltration. Am J Obstet Gynecol 157: 1275, 1987 |
|
DePraeter C, Vanhaesebrouch P, DePraeter N et al: Epistotomy and neonatal lidocaine intoxication [letter]. Eur J Pediatr 150: 685, 1991 |
|
Hill JB, Alexander JM, Sharma SK et al: A comparison of the effects of epidural and meperidine analgesia during labor onfetal heart rate. Obstet Gynecol. 2003 Aug;102(2):333-7. |
|
McIntosh DG, Rayburn WF: Patient-controlled analgesia in obstetrics and gynecology. Obstet Gynecol 78: 1129, 1991 |
|
Rayburn W, Leushcen MP, Earl R et al: Intravenous meperidine during labor: A randomized comparison between nursing and patient-controlled administration. Obstet Gynecol 74: 702, 1989 |
|
Podlas J, Breland BD: Patient-controlled analgesia with nalbuphine during labor. Obstet Gynecol 70: 202, 1987 |
|
Nikkola EM, Ekblad UU, Kerno PO et al: Intravenous fentanyl PCA during labour. Can J Anaesth 44: 1248, 1997 |
|
Balki M, Kasodekar S, Dhumne S et al: Remifentanil patient-controlled analgesia for labour: optimizing drug deliveryregimens. Can J Anaesth. 2007 Aug;54(8):626-33. |
|
Volikas I, Butwick A, Wilkinson C et al: Maternal and neonatal side-effects of remifentanil patient-controlled analgesiain labour. Br J Anaesth. 2005 Oct;95(4):504-9. |
|
Volmanen P, Akural E, Raudaskoski T et al: Comparison of remifentanil and nitrous oxide in labour analgesia. Acta Anaesthesiol Scand. 2005 Apr;49(4):453-8. |
|
Blair JM, Dobson GT, Hill DA et al: Patient controlled analgesia for labour: a comparison of remifentanil withpethidine. Anaesthesia. 2005 Jan;60(1):22-7. |
|
Hill D: Remifentanil patient-controlled analgesia should be routinely available for usein labour. Int J Obstet Anesth. 2008 Oct;17(4):336-9. |
|
Rosen M: Recent advances in pain relief in childbirth: Inhalation and systemic analgesia. Br J Anaesth 43: 837, 1971 |
|
Stefani SJ, Hughes SC, Shnider SM et al: Neonatal neurobehavioral effects of inhalation analgesia for vaginal delivery. Anesthesiology 56: 351, 1982 |
|
Deckardt R, Fembacher PM, Schneider KT et al: Maternal arterial oxygen saturation during labor and delivery: Pain dependent alteration and effects on the newborn. Obstet Gynecol 70: 21, 1987 |
|
Reed PN, Colquhoun AD, Hanning CD: Maternal oxygenation during normal labour. Br J Anaesth 62: 316, 1989 |
|
Mills GH, Singh D, Longan M et al: Nitrous oxide exposure on the labour ward. Int J Obstet Anesth 5: 160, 1996 |
|
Fernando R, Jones T. Systemic analgesia: Parenteral and inhalational agents. In Chestnut DH, Polley LS, Tsen LC, Wong CA (eds): Obstetric Anesthesia: Principles and Practice, 4th ed. pp. 415-27. Philadelphia: Elsevier Mosby 2009. |
|
Lindell SG: Education for childbirth: A time for change. J Obstet Gynecol Neonatal Nurs 17: 108, 1988 |
|
Minnich ME. Childbirth preparation and nonpharmacologic analgesia. In Chestnut DH, Polley LS, Tsen LC, Wong CA, eds. Obstetric Anesthesia Principles and Practice, 4th ed. Philadelphia: Elsevier Mosby 2009:405-14. |
|
Kennell J, Klaus M, McGrath S et al: Continuous emotional support during labor in a US hospital: A randomized controlled trial. JAMA 265: 2197, 1991 |
|
Hofmeyr GJ, Nikodem VC, Wolman W et al: Companionship to modify the clinical birth environment: Effects on progress and perceptions of labour and breastfeeding. Br J Obstet Gynaecol 98: 756, 1991 |
|
Huntley AL, Coon JT, Ernst E: Complementary and alternative medicine for labor pain: a systematic review. Am J Obstet Gynecol. 2004 Jul;191(1):36-44. |
|
Hawkins JL, Chang J, Palmer SK, Callaghan WM, Gibbs CP. Anesthesia-related maternal mortality in the United States, 1997-2002 [abstract]. Presented at the 2008 American Society of Anesthesiologists Annual Meeting, Orlando, FL. http://www.asaabstracts.com/strands/asaabstracts/printAbstract.htm;jsessionid=714E6BB3A3BEA4970DB7EB37D350B00C?index=3&year=2008&absnum=235&type=search. Assessed October 2, 2009. |
|
Conklin KA: Can anesthetic-related maternal mortality be reduced? Am J Obstet Gynecol 163: 253, 1990 |
|
Morgan BM, Magni V, Goroszenuik T: Anaesthesia for emergency Cesarean section. Br J Obstet Gynaecol 97: 420, 1990 |
|
Lambert DH, Hurley RJ, Hertwig L et al: Role of needle gauge and tip configuration in the production of lumbar puncture headache. Reg Anesth 22: 66, 1997 |
|
Reynolds F, Seed PT: Anaesthesia for Caesarean section and neonatal acid-base status: a meta-analysis. Anaesthesia. 2005 Jul;60(7):636-53. |
|
Ngan Kee WD, Khaw KS, Tan PE et al: Placental transfer and fetal metabolic effects of phenylephrine and ephedrineduring spinal anesthesia for cesarean delivery. Anesthesiology. 2009 Sep;111(3):506-12. |
|
Dahlgren G, Hulstrand C, Jakobsson J et al: Intrathecal sufentanil, fentanyl, or placebo added to bupivacaine for Cesarean section. Anesth Analg 85: 1288, 1997 |
|
DiFazio CA, Carron H, Grosslight KR et al: Comparison of pH-adjusted lidocaine solutions for epidural anesthesia. Anesth Analg 65: 760, 1986 |
|
Kangas-Saarela T, Jouppila R, Alahuhta S: The effect of lumbar epidural analgesia on neurobehavioral responses of newborn infants. Acta Anaesthesiol Scand 33: 320, 1989 |
|
Gaffud MP, Bansal P, Lawton C et al: Surgical analgesia for Cesarean delivery with epidural bupivacaine and fentanyl. Anesthesiology 65: 331, 1986 |
|
Preston PG, Rosen MA, Hughs SC et al: Epidural anesthesia with fentanyl and lidocaine for Cesarean section: Maternal effects and neonatal outcome. Anesthesiology 68: 938, 1988 |
|
Davies SJ, Paech MJ, Welch H et al: Maternal experience during epidural or combined spinal-epidural anesthesia for Cesarean section: A prospective, randomized trial. Anesth Analg 85: 607, 1997 |
|
Fan SZ, Susetio L, Wang YP et al: Low dose of intrathecal hyperbaric bupivacaine combined with epidural lidocaine for Cesarean section. Anesth Analg 78: 474, 1994 |
|
Datta S, Ostheimer GW, Weiss JB et al: Neonatal effect of prolonged anesthetic induction for Cesarean section. Obstet Gynecol 58: 331, 1981 |
|
Ong BY, Cohen MM, Palahniuk RJ: Anesthesia for Cesarean section—effects on neonates. Anesth Analg 68: 270, 1989 |
|
Cohen SE, Subak LL, Brose WG et al: Analgesia after cesarean delivery: Patient evaluations and costs of five opioid techniques. Reg Anesth 16: 141, 1991 |
|
Eisenach JC, Grice SC, Dewan DM: Patient-controlled analgesia following cesarean section: A comparison with epidural and intramuscular narcotics. Anesthesiology 68: 444, 1988 |
|
Yarnell RW, Polis T, Reid GN et al: Patient-controlled analgesia with epidural meperidine after elective Cesarean section. Reg Anesth 17: 329, 1992 |
|
Harrison DM, Sinatra R, Morgese L et al: Epidural narcotic and patient-controlled analgesia for post-Cesarean section pain relief. Anesthesiology 68: 454, 1988 |
|
Smith CV, Rayburn WF, Karaiskakis PT et al: Comparison of patient-controlled analgesia and epidural morphine for postcesarean pain and recovery. J Reprod Med 36: 430, 1991 |
|
Paech MJ, Moore JS, Evans SF: Meperidine for patient-controlled analgesia after Cesarean section. Intravenous versus epidural administration. Anesthesiology 80: 1268, 1994 |
|
Cade L, Ashley J: Towards optimal analgesia after caesarean section: comparison of epidural and intravenous patient-controlled opioid analgesia. Anaesth Intensive Care 21: 416, 1993 |
|
Parker RK, White PF: Epidural patient-controlled analgesia: An alternative to intravenous patient-controlled analgesia for pain relief after cesarean delivery. Anesth Analg 75: 245, 1992 |
|
Rawal N, Sjostrand U, Christoffersson E: Comparison of intramuscular and epidural morphine for postoperative analgesia in the grossly obese: Influence on postoperative ambulation and pulmonary function. Anesth Analg 63: 583, 1984 |
|
Husaini SW, Russel IF: Intrathecal diamorphine compared with morphine for postoperative analgesia after Caesarean section under spinal anaesthesia. Br J Anaesth 81: 135, 1998 |
|
Kelly MC, Carabine UA, Mirakhur RK: Intrathecal diamorphine for analgesia after caesarean section: A dose finding study and assessment of side-effects. Anaesthesia 53: 231, 1998 |
|
Youngstrom P, Hoyt M, Herman M: Dose-response study of continuous infusion epidural fentanyl-epinephrine for postcesarean analgesia. Anesthesiology 73: A984, 1990 |
|
Cohen S, Amar D, Pantuck CB: Postcesarean delivery epidural patient-controlled analgesia, fentanyl or sufentanil. Anesthesiology 78: 486, 1993 |
|
Brose WG, Cohen SE: Oxyhemoglobin saturation following cesarean section in patients receiving epidural morphine, PCA, or in meperidine analgesia. Anesthesiology 70: 948, 1989 |
|
Kafer ER, Brown JT, Scott DD: Biphasic depression of ventilatory responses to CO2 following epidural morphine. Anesthesiology 58: 418, 1987 |
|
Abouleish E, Rashad MN, Rawal N: The addition of 0.2 mg subarachnoid morphine to hyperbaric bupivacaine for cesarean delivery: A prospective study of 856 cases. Reg Anesth 16: 137, 1991 |
|
Horlocker TT, Burton AW, Connis RT et al: Practice guidelines for the prevention, detection, and management of respiratorydepression associated with neuraxial opioid administration. Anesthesiology. 2009 Feb;110(2):218-30. |
|
Cardoso MMSC, Carvalho JCA, Amaro AR: Small doses of intrathecal morphine combined with systemic diclofenac for post operative pain control after cesarean delivery. Anesth Analg 86: 538, 1998 |
|
Dennis AR, Leeso-Payne CG, Hobbs GJ: Analgesia after caesarean section. The use of rectal diclofenac as an adjunct to spinal morphine. Anaesthesia 50: 297, 1995 |
|
Pavy TJG, Gambling DR, Merrick PM et al: Rectal indomethacin potentiates spinal morphine analgesia after Caesarean delivery. Anaesth Intens Care 23: 555, 1995 |
|
Roseag OP, Lui ACP, Cicutti NJ et al: Peri-operative multi-modal pain therapy for caesarean section: Analgesia and fitness for discharge. Can J Anaesth 44: 803, 1997 |
|
Sun HL, Wu CC, Lin MS et al: Effects of epidural morphine and intramuscular diclofenac combination in postcesarean analgesia: A dose-range study. Anesth Analg 76: 284, 1993 |
|
McDonnell JG, Curley G, Carney J et al: The analgesic efficacy of transversus abdominis plane block after cesareandelivery: a randomized controlled trial. Anesth Analg. 2008 Jan;106(1):186-91. |
|
Rayburn WF, Geranis BJ, Ramadei CA et al: Patient-controlled analgesia for post-Cesarean pain. Obstet Gynecol 72: 136, 1988 |
|
Wittels B, Glosten B, Faure EA et al: Postcesarean analgesia with both epidural morphine and intravenous patient-controlled analgesia: Neurobehavioral outcomes among nursing neonates. Anesth Analg 85: 600, 1997 |
|
Parker RK, Holtmann B, White PF: Patient-controlled analgesia: Does a concurrent opioid infusion improve pain management after surgery? JAMA 266: 1947, 1991 |
|
Parker RK, Holtmann B, White PF: Effects of a nighttime opioid infusion with PCA therapy on patient comfort and analgesic requirements after abdominal hysterectomy. Anesthesiology 72: 362, 1992 |
|
McKenzie R: Patient-controlled analgesia (PCA) [letter]. Anesthesiology 69: 1027, 1988 |
|
Hepner D, Eappen S. Postoperative analgesia: Systemic and local techniques. In Chestnut DH, Polley LS, Tsen LC, Wong CA (eds): Obstetric Anesthesia: Principles and Practice, 4th ed. pp. 575-92. Philadelphia: Elsevier Mosby 2009. |
|
Dufour P, Vinatier D, Orazi G et al: The use of intravenous nitroglycerin for emergency cervico-uterine relaxation. Acta Obstet Gynecol Scand 76: 287, 1997 |
|
Greenspoon JS, Kovacic A: Breech extraction facilitated by glyceryl trinitrate sublingual spray. Lancet 13: 124, 1991 |
|
Rolbin S, Hew E, Bernstein A: Uterine relaxation can be life saving. Can J Anaesth 38: 939, 1991 |
|
Wessen A, Elowsson P, Axemo P et al: The use of intravenous nitroglycerin for emergency cervico-uterine relaxation. Acta Anaesthesiol Scand 39: 847, 1995 |
|
Crawford JS: A prospective study of 200 consecutive twin deliveries. Anaesthesia. 1987 Jan;42(1):33-43. |
|
Williams KP, Galerneau F: Intrapartum influences on cesarean delivery in multiple gestation. Acta Obstet Gynecol Scand. 2003 Mar;82(3):241-5. |
|
Johnson C, Oriol N: The role of epidural anesthesia in trial of labor. Reg Anesth 15: 304, 1990 |
|
Case BD, Corcoran R, Jeffcoate N et al: Caesarean section and its place in modern obstetric practice. J Obstet Gynaecol Br Commonw 78: 203, 1971 |
|
Carlsson C, Nybell-Lindahl G: Extradural block in patients who have previously undergone Caesarean section. Br J Anaesth 52: 827, 1980 |
|
Kelly MC, Hill DA, Wilson DB: Low dose epidural bupivacaine/fentanyl infusion does not mask uterine rupture. Int J Obstet Anesth 6: 52, 1997 |
|
Rowbottom SJ, Tabrizian I: Epidural analgesia and uterine rupture during labour. Anaesth Intens Care 22: 79, 1994 |
|
Rowbottom SJ, Critchley LAH, Gin T: Uterine rupture and epidural analgesia during trial of labour. Anaesthesia 52, 1997 |
|
Uppington J: Epidural analgesia and previous Caesarean section. Anaesthesia 38: 336, 1983 |
|
Flamm BL, Lim OW, Jones C et al: Vaginal birth after cesarean section: Results of a multicenter study. Am J Obstet Gynecol 158: 1079, 1988 |
|
Phelan JP, Clark SL, Diaz F et al: Vaginal birth after cesarean. Am J Obstet Gynecol 157: 1510, 1987 |
|
ACOG Practice Bulletin #54: vaginal birth after previous cesarean. Obstet Gynecol. 2004 Jul;104(1):203-12. |
|
Weiner CP: The clinical spectrum of preeclampsia. Am J Kidney Dis 9: 312, 1987 |
|
Kelton JG, Hunter DJ, Neame PB: A platelet function defect in pre-eclampsia. Obstet Gynecol 65: 107, 1985 |
|
Ramanathan J, Sibai BM, Vu T et al: Correlation between bleeding times and platelet counts in women with pre-eclampsia undergoing Cesarean section. Anesthesiology 71: 188, 1989 |
|
Beilin Y, Bodian CA, Haddad EM et al: Practice patterns of anesthesiologists regarding situations in obstetric anesthesia where clinical management is controversial. Anesth Analg 83: 735, 1996 |
|
Vandermeulen EP, Van Aken H, Vermylen J: Anticoagulants and spinal-epidural anesthesia. Anesth Analg 79: 1165, 1994 |
|
Leduc L, Wheeler JM, Kirshon B: Coagulation profile in severe preeclampsia. Obstet Gynecol 79: 14, 1992 |
|
Vincent RD, Chestnut DH, Sipes SL et al: Magnesium sulfate decreases maternal blood pressure but not uterine blood flow during epidural anesthesia in gravid ewes. Anesthesiology 74: 77, 1991 |
|
Kussmann B, Shorten G, Uppington J et al: Administration of magnesium sulphate before rocuronium: Effects on speed of onset and duration of neuromuscular block. Br J Anaesth 79: 122, 1997 |
|
Polley LS. Hypertensive disorders. In Chestnut DH, Polley LS, Tsen LC, Wong CA (eds): Obstetric Anesthesia: Principles and Practice, 4th ed. pp. 975-1007. Philadelphia: Elsevier Mosby 2009. |
|
Ramanathan J, Coleman P, Sibai B: Anesthetic modification of hemodynamic and neuroendocrine stress responses to cesarean delivery in women with severe pre-eclampsia. Anesth Analg 73: 772, 1991 |
|
Brimacombe J: Acute pharyngolaryngeal oedema and pre-eclamptic toxaemia. Anaesth Intens Care 20: 97, 1992 |
|
Hood DD, Curry R: Spinal versus epidural anesthesia for cesarean section in severely preeclamptic patients: A retrospective survey. Anesthesiology 90: 1276, 1999 |
|
Wallace DH, Leveno KJ, Cunningham FG et al: Randomized comparison of general and regional anesthesia for cesarean delivery inpregnancies complicated by severe preeclampsia. Obstet Gynecol. 1995 Aug;86(2):193-9. |
|
Aya AG, Mangin R, Vialles N et al: Patients with severe preeclampsia experience less hypotension during spinalanesthesia for elective cesarean delivery than healthy parturients: a prospectivecohort comparison. Anesth Analg. 2003 Sep;97(3):867-72. |
|
Aya AG, Vialles N, Tanoubi I et al: Spinal anesthesia-induced hypotension: a risk comparison between patients withsevere preeclampsia and healthy women undergoing preterm cesarean delivery. Anesth Analg. 2005 Sep;101(3):869-75. |
|
Dyer RA, Els I, Farbas J et al: Prospective, randomized trial comparing general with spinal anesthesia forcesarean delivery in preeclamptic patients with a nonreassuring fetal hearttrace. Anesthesiology. 2003 Sep;99(3):561-9. |
|
Vincent RD, Chestnut DH, Sipes SL et al: Epidural anesthesia worsens uterine blood flow and fetal oxygenation during hemorrhage in gravid ewes. Anesthesiology 76: 799, 1992 |
|
Bonner SM, Haynes SR, Ryall D: The anaesthetic management of Cesarean section for placenta previa: A questionnaire survey. Anaesthesia 50: 992, 1995 |
|
Chattopadhyay SK, Kharif H, Sherbeeni MM: Placenta praevia and accreta after previous Caesarean section. Eur J Obstet Gynecol Reprod Biol 52: 151, 1993 |
|
Chestnut DH, Redick LF: Continuous epidural anesthesia for elective cesarean hysterectomy. South Med J 78: 1168, 1985 |
|
Chestnut DH, Dwarn DM, Redick LF et al: Anesthetic management for obstetric hysterectomy: A multi-institutional study. Anesthesiology 70: 607, 1989 |
|
Frederiksen MC, Glassenberg R, Stika CS: Placenta previa: A 22-year analysis. Am J Obstet Gynecol 180: 1432, 1999 |
|
Bhavani-Shankar K, Lynch EP, Datta S: Airway changes during Cesarean hysterectomy. Can J Anaesth 47: 338, 2000 |
|
Clark SL, Belfort MA, Dildy GA et al: Maternal death in the 21st century: causes, prevention, and relationship tocesarean delivery. Am J Obstet Gynecol. 2008 Jul;199(1):36.e1-5. |
|
Horlocker TT, Wedel DJ, Benzon H et al: Regional anesthesia in the anticoagulated patient: defining the risks (the secondASRA Consensus Conference on Neuraxial Anesthesia and Anticoagulation). Reg Anesth Pain Med. 2003 May-Jun;28(3):172-97. |
|
Royal College of Obstetricians and Gynaecologists. Guideline No. 37: Thromboprophylaxis during pregnancy, labour and after vaginal delivery. 2004. Date accessed: September 30, 2009. Available from: http://www.aagbi.org/publications/guidelines/docs/infection_control_08.pdf. |
|
Pritchard JA: Haematological problems associated with delivery, placental abruption, retained dead fetus, and amniotic fluid embolism. Clin Haematol 2: 563, 1973 |
|
Fretts RC, Boyd ME, Usher RH et al: The changing pattern of fetal death, 1961-1988. Obstet Gynecol 79: 25, 1992 |
|
Laudenbach V, Mercier FJ, Roze JC et al: Anaesthesia mode for caesarean section and mortality in very preterm infants: anepidemiologic study in the EPIPAGE cohort. Int J Obstet Anesth. 2009 Apr;18(2):142-9. |
|
Crawford JS: Some maternal complications of epidural analgesia for labour. Anaesthesia 40: 1219, 1985 |
|
Hellmann K: Epidural anesthesia in obstetrics: A second look at 26,127 cases. Can Anaesth Soc J 12: 398, 1965 |
|
Paech MJ, Godkin R, Webster GS: Complications of obstetric epidural analgesia and anaesthesia: A prospective analysis of 10,995 cases. Int J Obstet Anesth 7: 5, 1998 |
|
Scott DB, Hibbard BM: Serious non-fatal complications associated with extradural block in obstetric practice. Br J Anaesth 64: 537, 1990 |
|
Moen V, Dahlgren N, Irestedt L: Severe neurological complications after central neuraxial blockades in Sweden Anesthesiology. 2004 Oct;101(4):950-9. |
|
Blanco JD, Gibbs RS, Casteneda YS: Bacteremia in obstetrics: Clinical course. Obstet Gynecol 58: 621, 1981 |
|
Bader AM, Gilbertson L, Kirz L et al: Regional anesthesia in women with chorioamnionitis. Reg Anesth 17: 84, 1992 |
|
Goodman EJ, DeHorta E, Taguiam JM: Safety of spinal and epidural anesthesia in parturients with chorioamnionitis. Reg Anesth 21: 436, 1996 |
|
Carp H, Bailey S: The association between meningitis and dural puncture in bacteremic rats. Anesthesiology 76: 739, 1992 |
|
Segal S. Fever and infection. In Chestnut DH, Polley LS, Tsen LC, Wong CA (eds): Obstetric Anesthesia: Principles and Practice, 4th ed. pp. 795-810. Philadelphia: Elsevier Mosby 2009. |
|
Lyons G: Failed intubation. Six years' experience in a teaching maternity unit. Anaesthesia 40: 759, 1985 |
|
Rocke DA, Murray WB, Rout CC et al: Relative risk analysis of factors associated with difficult intubation in obstetric anesthesia. Anesthesiology 77: 67, 1992 |
|
American College of Obstetricians and Gynecologists: Committee opinion: Anesthesia for emergency deliveries. No. 104, March 1992 |
|
Practice guidelines for management of the difficult airway: an updated report bythe American Society of Anesthesiologists Task Force on Management of theDifficult Airway. Anesthesiology. 2003 May;98(5):1269-77. |
|
Malignant Hyperthermia Association of the United States. Emergency therapy for malignant hyperthermia. http://medical.mhaus.org/PubData/PDFs/treatmentposter.pdf. Accessed October 4, 2009. |
|
Schorr SJ, Speights SE, Ross EL et al: A randomized trial of epidural anesthesia to improve external cephalic version success. Am J Obstet Gynecol 177: 1133, 1997 |
|
Weiniger CF, Ginosar Y, Elchalal U et al: External cephalic version for breech presentation with or without spinalanalgesia in nulliparous women at term: a randomized controlled trial. Obstet Gynecol. 2007 Dec;110(6):1343-50. |
|
Mancuso KM, Yancey MK, Murphy JA et al: Epidural analgesia for cephalic version: a randomized trial. Obstet Gynecol. 2000 May;95(5):648-51. |
|
Dugoff L, Stamm CA, Jones OW et al: The effect of spinal anesthesia on the success rate of external cephalic version: A randomized trial. Obstet Gynecol 93: 345, 1999 |
|
Sullivan JT, Grobman WA, Bauchat JR et al: A randomized controlled trial of the effect of combined spinal-epidural analgesiaon the success of external cephalic version for breech presentation. Int J Obstet Anesth. 2009 Oct;18(4):328-34. |
|
Macarthur AJ, Gagnon S, Tureanu LM et al: Anesthesia facilitation of external cephalic version: a meta-analysis. Am J Obstet Gynecol. 2004 Oct;191(4):1219-24. |
|
Neiger R, Hennessy MD, Patel M: Reattempting failed external cephalic version under epidural anesthesia. Am J Obstet Gynecol. 1998 Nov;179(5):1136-9. |
|
Cherayil G, Feinberg B, Robinson J et al: Central neuraxial blockade promotes external cephalic version success after afailed attempt. Anesth Analg. 2002 Jun;94(6):1589-92. |
|
American College of Obstetricians and Gynecologists: External cephalic version. ACOG Practice Patterns, No. 4, Washington, DC, 1997 |
|
Chantigian RC, Chantigian PDM. Problems of early pregnancy. In Chestnut DH, Polley LS, Tsen LC, Wong CA (eds): Obstetric Anesthesia: Principles and Practice, 4th ed. pp. 319-35. Philadelphia: Elsevier Mosby 2009. |
|
Crawford JS, Lewis M: Nitrous oxide in early human pregnancy. Anaesthesia 41: 900, 1986 |
|
DeSimone CA, Norris MC, Leighton BL: Intravenous nitroglycerin aids manual extraction of a retained placenta [letter]. Anesthesiology 73: 787, 1990 |
|
Peng AT, Gorman RS, Shulman SM et al: Intravenous nitroglycerin for uterine relaxation in the postpartum patient with retained placenta [letter]. Anesthesiology 71: 172, 1989 |
|
Vincent RD, Reid RW: Epidural anesthesia for postpartum tubal ligation using epidural catheters placed during labor. J Clin Anesth 5: 289, 1993 |
|
Lawlor M, Weinter M, Fantauzzi M et al: Efficacy of epidural anesthesia for post-partum tubal ligation utilizing indwelling labor epidural catheters. Reg Anesth 19: 54, 1994 |
|
Goodman EJ, Dumas SD: The rate of successful reactivation of labor epidural catheters for postpartum tubal ligation surgery. Reg Anesth Pain Med 23: 258, 1998 |
|
Marcus RJ, Wong CA, Lehor A et al: Postoperative epidural morphine for postpartum tubal ligation analgesia. Anesth Analg. 2005 Sep;101(3):876-81. |
|
Habib AS, Muir HA, White WD et al: Intrathecal morphine for analgesia after postpartum bilateral tubal ligation. Anesth Analg. 2005 Jan;100(1):239-43. |
|
Van de Velde M. Nonobstetric surgery during pregnancy. In Chestnut DH, Polley LS, Tsen LC, Wong CA (eds): Obstetric Anesthesia: Principles and Practice, 4th ed. pp. 337-60. Philadelphia: Elsevier Mosby 2009. |
|
Ralston DH, Shnider SM: The fetal and neonatal effects of regional anesthesia in obstetrics. Anesthesiology 48: 34, 1978 |
|
Brizgys RV, Dailey PA, Shnider SM et al: The incidence and neonatal effects of maternal hypotension during epidural anesthesia for Cesarean section. Anesthesiology 67: 782, 1987 |
|
Rout CC, Rocke DA, Levin J et al: A reevaluation of the role of crystalloid preload in the prevention of hypotension associated with spinal anesthesia for elective Cesarean section. Anesthesiology 79: 262, 1993 |
|
Tong C, Eisenach JC: The vascular mechanism of ephedrine's beneficial effect on uterine perfusion during pregnancy. Anesthesiology 76: 792, 1992 |
|
Lee A, Ngan Kee WD, Gin T: A quantitative, systematic review of randomized controlled trials of ephedrineversus phenylephrine for the management of hypotension during spinal anesthesiafor cesarean delivery. Anesth Analg. 2002 Apr;94(4):920-6. |
|
Hofmeyr G, Cyna A, Middleton P: Prophylactic intravenous preloading for regional analgesia in labour. Cochrane Database Syst Rev. 2004 Oct 18;(4):CD000175. |
|
Morgan PJ, Halpern SH, Tarshis J: The effects of an increase of central blood volume before spinal anesthesia forcesarean delivery: a qualitative systematic review. Anesth Analg. 2001 Apr;92(4):997-1005. |
|
Dyer RA, Farina Z, Joubert IA et al: Crystalloid preload versus rapid crystalloid administration after induction ofspinal anaesthesia (coload) for elective caesarean section. Anaesth Intensive Care. 2004 Jun;32(3):351-7. |
|
Ngan Kee WD, Khaw KS, Ng FF: Prevention of hypotension during spinal anesthesia for cesarean delivery: aneffective technique using combination phenylephrine infusion and crystalloidcohydration. Anesthesiology. 2005 Oct;103(4):744-50. |
|
Clarke VT, Smiley RM, Finster M: Uterine hyperactivity after intrathecal injection of fentanyl for analgesiaduring labor: a cause of fetal bradycardia? Anesthesiology. 1994 Oct;81(4):1083. |
|
Mardirosoff C, Dumont L, Boulvain M et al: Fetal bradycardia due to intrathecal opioids for labour analgesia: a systematicreview. BJOG. 2002 Mar;109(3):274-81. |
|
Mercier FJ, Dounas M, Bouaziz H et al: Intravenous nitroglycerin to relieve intrapartum fetal distress related touterine hyperactivity: a prospective observational study. Anesth Analg. 1997 May;84(5):1117-20. |
|
Lybecker H, Djemes M, Schmidt JF: Post-dural puncture headache (PDPH): Onset, duration, severity, and associated symptoms. An analysis of 75 consecutive patients with PDPH. Acta Anaesthesiol Scand 39: 605, 1995 |
|
Shearer VE, Jhaveri HS, Cunningham FG: Puerperal seizures after post-dural puncture headache. Obstet Gynecol 85: 255, 1995 |
|
Choi PT, Galinski SE, Takeuchi L et al: PDPH is a common complication of neuraxial blockade in parturients: a Can J Anaesth. 2003 May;50(5):460-9. |
|
Sechzer PH, Abel L: Post-spinal anesthesia headache treated with caffeine. Evaluation with demand method. Curr Ther Res 24: 307, 1978 |
|
Camann WR, Murray RS, Mushlin PS et al: Effects of oral caffeine on postdural puncture headache: A double-blind, placebo-controlled trial. Anesth Analg 70: 181, 1990 |
|
Williams EJ, Beaulieu P, Fawcett WJ et al: Efficacy of epidural blood patch in the obstetric population. Int J Obstet Anesth 8: 105, 1999 |
|
Taivainen T, Pitkanen M, Tuominen M et al: Efficacy of epidural blood patch for postdural puncture headache. Acta Anaesthesiol Scand 37: 702, 1993 |
|
Stride PC, Cooper PC: Dural taps revisited. A 20-year survey from Birmingham Maternity Hospital. Anaesthesia 48: 247, 1993 |
|
Abouleish E, de la Vega S, Blendinger TO: Long term follow-up of epidural blood patch. Anesth Analg 54: 459, 1975 |
|
Leighton BL, Halpern SH: The effects of epidural analgesia on labor, maternal, and neonatal outcomes: asystematic review. Am J Obstet Gynecol. 2002 May;186(5 Suppl Nature):S69-77. |
|
Goetzl L, Rivers J, Zighelboim I et al: Intrapartum epidural analgesia and maternal temperature regulation. Obstet Gynecol. 2007 Mar;109(3):687-90. |
|
Goetzl L, Zighelboim I, Badell M et al: Maternal corticosteroids to prevent intrauterine exposure to hyperthermia and Am J Obstet Gynecol. 2006 Oct;195(4):1031-7. |
|
Lieberman E, Lang JM, Frigoletto F Jr et al: Epidural analgesia, intrapartum fever, and neonatal sepsis evaluation. Pediatrics 99: 415, 1997 |
|
To WW, Wong MW: Factors associated with back pain symptoms in pregnancy and the persistence ofpain 2 years after pregnancy. Acta Obstet Gynecol Scand. 2003 Dec;82(12):1086-91. |
|
Loughnan BA, Carli F, Romney M et al: Epidural analgesia and backache: a randomized controlled comparison withintramuscular meperidine for analgesia during labour. Br J Anaesth. 2002 Sep;89(3):466-72. |
|
Howell CJ, Kidd C, Roberts W et al: A randomised controlled trial of epidural compared with non-epidural analgesia inlabour. BJOG. 2001 Jan;108(1):27-33. |
|
Howell CJ, Dean T, Lucking L et al: Randomised study of long term outcome after epidural versus non-epiduralanalgesia during labour. BMJ. 2002 Aug 17;325(7360):357. |
|
Albright GA: Cardiac arrest following regional anesthesia with etidocaine or bupivacaine. Anesthesiology 51: 285, 1979 |
|
Turner-Lawrence DE, Kerns Ii W: Intravenous fat emulsion: a potential novel antidote. J Med Toxicol. 2008 Jun;4(2):109-14. |
|
Marx GF: Cardiopulmonary resuscitation of late-pregnant women. Anesthesiology 56: 156, 1982 |
|
Reynolds F: Neurological infections after neuraxial anesthesia. Anesthesiol Clin. 2008 Mar;26(1):23-52. |
|
Schneeberger PM, Janssen M, Voss A: Alpha-hemolytic streptococci: a major pathogen of iatrogenic meningitis followinglumbar puncture. Case reports and a review of the literature. Infection. 1996 Jan-Feb;24(1):29-33. |
|
Rubin L, Sprecher H, Kabaha A et al: Meningitis following spinal anesthesia: 6 cases in 5 years. Infect Control Hosp Epidemiol. 2007 Oct;28(10):1187-90. |
|
Auroy Y, Narchi P, Messiah A et al: Serious complications related to regional anesthesia. Anesthesiology 87: 479, 1997 |
|
Reynolds F: Damage to the conus medullaris following spinal anaesthesia. Anaesthesia. 2001 Mar;56(3):238-47. |
|
Wong CA, Scavone BM, Dugan S, Smith JC, Prather H, Ganchiff JN, McCarthy RJ. Incidence of postpartum lumbosacral spinal and lower extremity nerve injuries. Obstet Gynecol. 2003;101(2):279-88. |